A) \[I{{F}_{3}}\]
B) \[PC{{l}_{3}}\]
C) \[N{{H}_{3}}\]
D) \[B{{F}_{3}}\]
Correct Answer: D
Solution :
[a]\[I{{F}_{3}}\]has bent-T geometry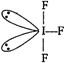 [b]\[PC{{l}_{3}}\]has pyramidal geometry
[b]\[PC{{l}_{3}}\]has pyramidal geometry 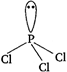 [c] \[N{{H}_{3}}\]has pyramidal geometry
[c] \[N{{H}_{3}}\]has pyramidal geometry 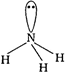 + [d] \[B{{F}_{3}}\] has trigonal planar geometry
+ [d] \[B{{F}_{3}}\] has trigonal planar geometry You need to login to perform this action.
You will be redirected in
3 sec
