question_answer 1) The internal resistance of a cell is the resistance of:
A)
electrolyte used in cell
done
clear
B)
electrodes of the cell
done
clear
C)
material used in cell
done
clear
D)
vessel of the cell
done
clear
View Answer play_arrow
question_answer 2) A device for generating an alternating current of d desired frequency is known as:
A)
an oscillator
done
clear
B)
an amplifier
done
clear
C)
a rectifier
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 3) The angle of minimum deviation for a thin prism with respect to air and when dipped in water will be:\[\left( _{a}{{\mu }_{g}}=\frac{3}{2},\,{{\,}_{a}}{{\mu }_{w}}=\frac{4}{3} \right)\]
A)
\[\frac{1}{4}\]
done
clear
B)
\[\frac{1}{8}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 4) Escape velocity of a body when projected from the earth's surface is \[11.2\,\,km/s\]. If it is projected at an angle of \[{{50}^{o}}\] from the horizontal, the escape velocity is:
A)
\[11.8\,\,km/s\]
done
clear
B)
\[16.5\,\,km/s\]
done
clear
C)
\[11.2\,\,km/s\]
done
clear
D)
\[14.5\,\,km/s\]
done
clear
View Answer play_arrow
question_answer 5) A diamagnetic substance is brought near a strong magnet, then it is:
A)
attracted by a magnet
done
clear
B)
repelled by a magnet
done
clear
C)
repelled by north pole and attracted by south pole
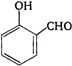
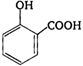
done
clear
D)
attracted by north pole and repelled by south pole
done
clear
View Answer play_arrow
question_answer 6) The ratio of intensities of two waves is \[9:16\]. If they interfere, the ratio of maximum to minimum intensity will be:
A)
\[4:1\]
done
clear
B)
\[1:25\]
done
clear
C)
\[1:3\]
done
clear
D)
\[49:1\]
done
clear
View Answer play_arrow
question_answer 7) The sun emits a light with maximum wavelength\[510\,\,nm\] while another star emits a light with maximum wavelength of \[350\,\,nm\]. The ratio of surface temperature of sun and the star will be:
A)
\[0.69\]
done
clear
B)
\[0.46\]
done
clear
C)
\[1.45\]
done
clear
D)
\[2.1\]
done
clear
View Answer play_arrow
question_answer 8) If the equation of Motion of standing wave is\[y=0.3\sin (314t-1.57x)\], then the velocity of standing wave is:
A)
\[400\,\,unit\]
done
clear
B)
\[350\,\,unit\]
done
clear
C)
\[209\,\,unit\]
done
clear
D)
\[200\,\,unit\]
done
clear
View Answer play_arrow
question_answer 9) For driving current of \[2\,\,A\] for \[6\min \] in a circuit, \[1000\,\,J\] of work is to be done. The emf-of the source in the circuit is:
A)
\[2.03\,\,V\]
done
clear
B)
\[2.54\,\,V\]
done
clear
C)
\[1.25\,\,V\]
done
clear
D)
\[1.39\,\,V\]
done
clear
View Answer play_arrow
question_answer 10) The moment of momentum for an electron in second orbit of hydrogen atom as per Bohr's model is:
A)
\[\frac{h}{\pi }\]
done
clear
B)
\[2\pi h\]
done
clear
C)
\[\frac{2h}{\pi }\]
done
clear
D)
\[\frac{\pi }{h}\]
done
clear
View Answer play_arrow
question_answer 11) The latent heat of vaporisation of water is\[2250\,\,J/kg\]. If the work done in the process of vaporisation of \[1\,\,kg\] is \[168\,\,J\], then increase in internal energy will be:
A)
\[1904\,\,J\]
done
clear
B)
\[1984\,\,J\]
done
clear
C)
\[3202\,\,J\]
done
clear
D)
\[2082\,\,J\]
done
clear
View Answer play_arrow
question_answer 12) Huygen's wave theory of light could not explain:
A)
photoelectric effect
done
clear
B)
interference
done
clear
C)
diffraction
done
clear
D)
polarization
done
clear
View Answer play_arrow
question_answer 13) In a triode valve, when the plate potential is increased from \[200\,\,V\] to \[220\,\,V\] and-grid potential is decreased from \[-0.5\,\,V\] to \[-\text{ }1.3\,\,V,\] there is no change in plate current. The amplification factor of the triode is:
A)
\[25\]
done
clear
B)
\[14\]
done
clear
C)
\[11\]
done
clear
D)
\[73\]
done
clear
View Answer play_arrow
question_answer 14) A bar magnet of magnetic moment \[220\,\,A{{m}^{2}}\] is suspended in a magnetic field of intensity\[0.25\,\,N/Am\]. The couple required to deflect it through \[{{30}^{o}}\] is:
A)
\[27.5\,\,Nm\]
done
clear
B)
\[20.25\,\,Nm\]
done
clear
C)
\[47.63\,\,Nm\]
done
clear
D)
\[12\,\,Nm\]
done
clear
View Answer play_arrow
question_answer 15) A plano-convex lens has refractive index \[1.5\] and radius of curvature \[50\,\,cm\]. What is focal length of lens?
A)
\[100\,\,cm\]
done
clear
B)
\[200\,\,cm\]
done
clear
C)
\[178\,\,cm\]
done
clear
D)
\[150\,\,cm\]
done
clear
View Answer play_arrow
question_answer 16) A source and an observer move away from each other, with a velocity of \[20\,\,m/s\]. If the apparent frequency heard by the observer is \[1840\,\,Hz\], the actual frequency of the source is: (Velocity of sound in\[=340\,\,m/s)\]:
A)
\[2486\,\,Hz\]
done
clear
B)
\[2070\,\,Hz\]
done
clear
C)
\[2134\,\,Hz\]
done
clear
D)
\[1872\,\,Hz\]
done
clear
View Answer play_arrow
question_answer 17) The speed of wave in a medium is \[650\,\,m/s\]. If \[3500\] waves are passing through a point in the medium in \[1.67\,\,\min \], then its wavelength will be:
A)
\[16.25\,\,m\]
done
clear
B)
\[14.29\,\,m\]
done
clear
C)
\[18.57\,\,m\]
done
clear
D)
\[20.50\,\,m\]
done
clear
View Answer play_arrow
question_answer 18) The body is projected at such angle that the horizontal range is three times the greatest height. The angle of projection is:
A)
\[{{43}^{o}}8'\]
done
clear
B)
\[{{25}^{o}}8'\]
done
clear
C)
\[{{33}^{o}}7'\]
done
clear
D)
\[{{53}^{o}}1'\]
done
clear
View Answer play_arrow
question_answer 19) On the horizontal surface of a truck, a block of mass \[1\,\,kg\] is placed \[(\mu =0.6)\] and truck is moving with acceleration \[5m/{{s}^{2}}\]. The frictional force acting on the block will be: \[(g=10\,\,m/{{s}^{2}})\]
A)
\[6\,\,N\]
done
clear
B)
\[5.88\,\,N\]
done
clear
C)
\[7\,\,N\]
done
clear
D)
\[9\,\,N\]
done
clear
View Answer play_arrow
question_answer 20) Calculate the work done when a force\[\overset{\to }{\mathop{\mathbf{F}}}\,=2\widehat{\mathbf{i}}+3\widehat{\mathbf{j}}-5\widehat{\mathbf{k}}\]. units acts on a body producing a displacement \[\overset{\to }{\mathop{\mathbf{s}}}\,=2\widehat{\mathbf{i}}+4\widehat{\mathbf{j}}+3\widehat{\mathbf{k}}\] units:
A)
\[1\,\,unit\]
done
clear
B)
\[20\,\,unit\]
done
clear
C)
\[5\,\,unit\]
done
clear
D)
\[zero\]
done
clear
View Answer play_arrow
question_answer 21) The efficiency of Carnot engine is, \[50%\] and temperature of sink is \[500\,\,K\]. If the temperature of source is kept constant and its efficiency is to be raised to \[60%\], then the required temperature of the sink will be:
A)
\[600\,\,K\]
done
clear
B)
\[400\,\,K\]
done
clear
C)
\[500\,\,K\]
done
clear
D)
\[100\,\,K\]
done
clear
View Answer play_arrow
question_answer 22) What happens to the internal energy of a gas during isothermal expansion?
A)
Internal energy will decrease
done
clear
B)
Internal energy will increase
done
clear
C)
Internal energy will become zero
done
clear
D)
Internal energy will remain same
done
clear
View Answer play_arrow
question_answer 23) \[100\,\,g\] ice is mixed with \[100\,\,g\] of water at \[{{100}^{o}}C\]. What will be the final temperature of the mixture?
A)
\[{{10}^{o}}C\]
done
clear
B)
\[{{27}^{o}}C\]
done
clear
C)
\[{{14}^{o}}C\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 24) Black hole consists of:
A)
ozone layer
done
clear
B)
super dense planetary material
done
clear
C)
upper surface of atmosphere
done
clear
D)
none of the above
done
clear
View Answer play_arrow
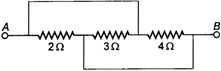
question_answer 25)
Three resistors \[2\,\,\Omega ,\,\,\,3\,\,\Omega \] and \[4\,\,\Omega \] are connected as shown in the given diagram. The equivalent resistance will be:
A)
\[\frac{12}{13}\]
done
clear
B)
\[\frac{11}{10}\]
done
clear
C)
\[2\]
done
clear
D)
\[\frac{10}{11}\]
done
clear
View Answer play_arrow
question_answer 26) If an electron jumps from first orbit to third orbit of hydrogen atom it will:
A)
not loose energy
done
clear
B)
release energy
done
clear
C)
absorb energy
done
clear
D)
not gain energy
done
clear
View Answer play_arrow
question_answer 27) A body of mass \[10\,\,kg\] moving with velocity \[10\,\,m/s\] collides with a stationary body of mass\[5\,\,kg\]. After collision both bodies stick to each other, velocity of the joint body after collision is:
A)
\[\frac{3}{10}m/s\]
done
clear
B)
\[20\,\,m/s\]
done
clear
C)
\[\frac{20}{3}m/s\]
done
clear
D)
\[15\,\,m/s\]
done
clear
View Answer play_arrow
question_answer 28) If a body starts from rest and travels \[110\,\,cm\] in the \[9th\] second, then acceleration of the body is:
A)
\[0.13m/{{s}^{2}}\]
done
clear
B)
\[0.16m/{{s}^{2}}\]
done
clear
C)
\[0.18m/{{s}^{2}}\]
done
clear
D)
\[0.34m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 29) It the momentum of a particle is doubled, then its de-Broglie wavelength will:
A)
become four times
done
clear
B)
become half
done
clear
C)
become two times
done
clear
D)
remain unchanged
done
clear
View Answer play_arrow
question_answer 30) Dimensions of torque are:
A)
\[[{{M}^{2}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-1}}]\]
done
clear
D)
\[[M{{L}^{0}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 31) Two vectors have magnitudes \[3\] and \[5\]. If angle between them is \[{{60}^{o}}\], then the dot product of two vectors will be:
A)
\[7.5\]
done
clear
B)
\[6.5\]
done
clear
C)
\[8.4\]
done
clear
D)
\[7.9\]
done
clear
View Answer play_arrow
question_answer 32) The kinetic energy of \[1\,\,g\] molecule of a gas, at normal temperature and pressure is:\[(R=8.321\,\,J/mol\text{-}K)\]
A)
\[1.2\times {{10}^{2}}J\]
done
clear
B)
\[3.4\times {{10}^{3}}J\]
done
clear
C)
\[1.66\times {{10}^{4}}J\]
done
clear
D)
\[2.97\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 33) If the coefficient of cubical expansion is \[x\] times of the coefficient of superficial expansion, then value of \[x\] is:
A)
\[2.7\]
done
clear
B)
\[2\]
done
clear
C)
\[1.5\]
done
clear
D)
\[9.5\]
done
clear
View Answer play_arrow
question_answer 34) \[X-\]ray will not show the phenomenon of:
A)
interference
done
clear
B)
deflection by electric field
done
clear
C)
diffraction
done
clear
D)
polarisation
done
clear
View Answer play_arrow
question_answer 35) Bernoulli's theorem is based on:
A)
conservation of mass, energy and momentum
done
clear
B)
conservation of mass
done
clear
C)
conservation of momentum
done
clear
D)
conservation of energy
done
clear
View Answer play_arrow
question_answer 36) In bringing an electron towards another electron, the electrostatic potential energy of the system:
A)
decreases
done
clear
B)
increases
done
clear
C)
remains same
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 37) The half-life of radium is \[1600\,\,yr\]. What is the mean life and disintegration constant of radium?
A)
\[2309\,\,yr,\,\,\frac{1}{2309}/yr\]
done
clear
B)
\[3309\,\,yr,\,\,\frac{1}{3309}/yr\]
done
clear
C)
\[1309\,\,yr,\,\,\frac{1}{1309}/yr\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 38) Energy obtained when \[1\,\,mg\] mass is completely converted in to energy will be:
A)
\[9\times {{10}^{13}}J\]
done
clear
B)
\[3\times {{10}^{8}}J\]
done
clear
C)
\[3\times {{10}^{15}}J\]
done
clear
D)
\[9\times {{10}^{15}}J\]
done
clear
View Answer play_arrow
question_answer 39) A ray of light is incident on a surface of a plate of glass of refractive index \[1.5\] at polarising angle. The angle of refraction of the ray will be:
A)
\[{{53.7}^{o}}\]
done
clear
B)
\[{{43.7}^{o}}\]
done
clear
C)
\[{{33.7}^{o}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 40) A closed organ pipe and an open organ pipe are tuned to the same fundamental frequency. The ratio of their lengths is:
A)
\[1:2\]
done
clear
B)
\[4:1\]
done
clear
C)
\[1:4\]
done
clear
D)
\[2:1\]
done
clear
View Answer play_arrow
question_answer 41) A particle is moving along a circular path of radius \[5\,\,m\] with uniform speed \[5\,\,m/s\]. What will be the average acceleration when the particle completes half revolution?
A)
\[\frac{10}{\pi }m/{{s}^{2}}\]
done
clear
B)
\[10\,\,m/{{s}^{2}}\]
done
clear
C)
\[10\pi \,\,m/{{s}^{2}}\]
done
clear
D)
\[Zero\]
done
clear
View Answer play_arrow
question_answer 42)
A body of mass \[m\] is attached between two springs of force constants \[{{k}_{1}}\] and \[{{k}_{2}}\] as Shown in figure. The other ends of the springs are fixed to firm supports. The frequency of the oscillation is:
A)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}-{{k}_{2}}}{m}}\]
done
clear
B)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}{{k}_{2}}}{m}}\]
done
clear
C)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}+{{k}_{2}}}{m}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 43) In a \[p-\]type semicoriductor, germanium is doped with:
A)
boron
done
clear
B)
gallium
done
clear
C)
aluminium
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 44) In a full wave rectifier circuit operating from \[50\,\,Hz\] mains frequency, what is the fundamental frequency in the ripple?
A)
\[50\,\,Hz\]
done
clear
B)
\[100\,\,Hz\]
done
clear
C)
\[25\,\,Hz\]
done
clear
D)
\[70\,\,Hz\]
done
clear
View Answer play_arrow
question_answer 45) An ideal gas at \[{{27}^{o}}C\] is compressed adiabatically to \[\frac{8}{27}\] of its original volume. The rise in temperature will be\[\left( \gamma =\frac{5}{3} \right)\].
A)
\[{{480}^{o}}C\]
done
clear
B)
\[{{275}^{o}}C\]
done
clear
C)
\[{{450}^{o}}C\]
done
clear
D)
\[{{375}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 46) When you make ice cubes, entropy of water:
A)
does not change
done
clear
B)
increases
done
clear
C)
decreases
done
clear
D)
may either increase or decrease depending on the process used
done
clear
View Answer play_arrow
question_answer 47) The focal length of the objective lens and eye-piece of an astronomical telescope are \[2\,\,m\] and\[0.05\,\,m\]. Find the length of the telescope.
A)
\[2.05\,\,m\]
done
clear
B)
\[1.16\,\,m\]
done
clear
C)
\[1.05\,\,m\]
done
clear
D)
\[2.9\,\,m\]
done
clear
View Answer play_arrow
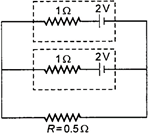
question_answer 48)
Two identical batteries, each of emf \[2\,\,V\] and internal resistance \[1\Omega \] pass a current through external, resistance\[R=0.5\Omega \]. The maximum power that can be developed across \[R\] using these batteries is:
A)
\[3.2\,\,W\]
done
clear
B)
\[8.2\,\,W\]
done
clear
C)
\[4\,\,W\]
done
clear
D)
\[2\,\,W\]
done
clear
View Answer play_arrow
question_answer 49) At what height from the earth's surface, the acceleration due to gravity will be half the value of \[g\] at surface? \[(R=6400\,\,km)\]
A)
\[6400\,\,km\]
done
clear
B)
\[8200\,\,km\]
done
clear
C)
\[4800\,\,km\]
done
clear
D)
\[1600\,\,km\]
done
clear
View Answer play_arrow
question_answer 50) In a semiconducting material the motilities of electrons and holes are \[{{\mu }_{e}}\] and \[{{\mu }_{h}}\] respectively which of the following is true?
A)
\[{{\mu }_{e}}>{{\mu }_{h}}\]
done
clear
B)
\[{{\mu }_{e}}<{{\mu }_{h}}\]
done
clear
C)
\[{{\mu }_{e}}={{\mu }_{h}}\]
done
clear
D)
\[{{\mu }_{e}}<0,\,\,{{\mu }_{h}}>0\]
done
clear
View Answer play_arrow
question_answer 51) Tyndall effect is shown by:
A)
solution
done
clear
B)
precipitate
done
clear
C)
sol
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 52) In reaction,\[MnO_{4}^{-}+{{H}^{+}}+{{C}_{2}}O_{4}^{2-}\xrightarrow{{}}M{{n}^{2+}}\]\[+{{H}_{2}}O+C{{O}_{2}}\]What is happening?
A)
Reduction of\[Mn\]
done
clear
B)
Reduction of\[{{C}_{2}}O_{4}^{2-}\]
done
clear
C)
Oxidation of\[Mn\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 53) In which of the following ionic bond is present?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[CC{{l}_{4}}\]
done
clear
C)
\[HCl\]
done
clear
D)
\[BaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 54) Deficiency of vitamin \[E\] causes:
A)
scurvy
done
clear
B)
beri-beri
done
clear
C)
sterility
done
clear
D)
xerophthalmia
done
clear
View Answer play_arrow
question_answer 55) The reaction of Lucas reagent is fast with:
A)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 56) \[IUPAC\] name of the compound is: \[H-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-C{{H}_{2}}-COOH\]
A)
3-oxo-butanoic acid
done
clear
B)
4-oxo-propanoic acid
done
clear
C)
3-formyl-propanoic acid
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 57) Tollen's reagent is:
A)
alkaline \[CuS{{O}_{4}}\] solution
done
clear
B)
ammoniacal cuproxide solution
done
clear
C)
aqueous solution of sodium cupritartarate
done
clear
D)
ammoniacal \[AgN{{O}_{3}}\] solution
done
clear
View Answer play_arrow
question_answer 58) Epsom salt is:
A)
\[MgS{{O}_{4}}\cdot 2{{H}_{2}}O\]
done
clear
B)
\[BaS{{O}_{4}}\cdot 2{{H}_{2}}O\]
done
clear
C)
\[MgS{{O}_{4}}\cdot 7{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}\cdot 2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 59) Which of the following carbonate decompose most easily on heating?
A)
\[R{{b}_{2}}C{{O}_{3}}\]
done
clear
B)
\[{{K}_{2}}C{{O}_{3}}\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
\[MgC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 60) In an isothermal expansion of an ideal gas:
A)
\[W=0\]
done
clear
B)
\[\Delta E=0\]
done
clear
C)
\[q=0\]
done
clear
D)
\[\Delta V=0\]
done
clear
View Answer play_arrow
question_answer 61) The degree of hydrolysis of \[0.01\,\,M\,\,N{{H}_{4}}Cl\] is:\[({{K}_{h}}=2.5\times {{10}^{-9}})\]
A)
\[5\times {{10}^{-5}}\]
done
clear
B)
\[5\times {{10}^{-4}}\]
done
clear
C)
\[5\times {{10}^{-3}}\]
done
clear
D)
\[5\times {{10}^{-7}}\]
done
clear
View Answer play_arrow
question_answer 62) The number of gram equivalent of \[{{H}_{2}}S{{O}_{4}}\] in \[1000\,\,mL\,\,3M\] solution, is:
A)
\[3\]
done
clear
B)
\[6\]
done
clear
C)
\[4\]
done
clear
D)
\[1.5\]
done
clear
View Answer play_arrow
question_answer 63) The radioactive isotope used in cancer therapy, is:
A)
\[{{I}^{128}}\]
done
clear
B)
\[C{{o}^{60}}\]
done
clear
C)
\[C{{o}^{59}}\]
done
clear
D)
\[{{p}^{32}}\]
done
clear
View Answer play_arrow
question_answer 64) The expression of angular momentum of an electron in a Bohr's orbit is:
A)
\[\frac{n\,\,h}{3\,\,\pi }\]
done
clear
B)
\[\frac{n\,\,h}{2\,\,\pi }\]
done
clear
C)
\[\frac{h}{4\pi }\]
done
clear
D)
\[\sqrt{l(l+1)}\cdot \frac{h}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following is called Hofmann's bromamide reaction?
A)
\[C{{H}_{3}}CHN+2{{H}_{2}}O\xrightarrow{{}}C{{H}_{3}}COO+N{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}CN+4H\xrightarrow{Na/ErOH}C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}CON{{H}_{2}}\xrightarrow{B{{r}_{2}}/NaOH}C{{H}_{3}}N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}COCl+C{{H}_{3}}OH\xrightarrow{{}}\]\[C{{H}_{3}}COOC{{H}_{3}}+HCl\]
done
clear
View Answer play_arrow
question_answer 66) Which one among the following contains a phenolic \[-OH\] group?
A)
Oxalic acid
done
clear
B)
Formic acid
done
clear
C)
Picric acid
done
clear
D)
Citric acid
done
clear
View Answer play_arrow
question_answer 67) In the reaction, product \['X'\] is: \[C{{H}_{3}}-C\equiv CH+{{H}_{2}}O\xrightarrow{{{H}^{+}}/H{{g}^{2+}}}X\]
A)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}-C(OH)=CHOH\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 68) Which is an amphoteric oxide?
A)
\[MgO\]
done
clear
B)
\[{{K}_{2}}O\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[CuO\]
done
clear
View Answer play_arrow
question_answer 69) The oxidation number of \[S\] in\[{{H}_{2}}{{S}_{2}}{{O}_{8}}\] is:
A)
\[+6\]
done
clear
B)
\[-6\]
done
clear
C)
\[+4\]
done
clear
D)
\[+8\]
done
clear
View Answer play_arrow
question_answer 70) Bleaching powder is obtained by treating chlorine with:
A)
\[CaC{{O}_{3}}\]
done
clear
B)
\[Ca{{(OH)}_{2}}\]
done
clear
C)
\[CaO\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 71) For reaction,\[PC{{l}_{3}}(g)+C{{l}_{2}}(g)PC{{l}_{5}}(g)\]the value of\[{{K}_{c}}\]at\[{{250}^{o}}C\]is\[26\]. At the same temperature, the value of \[{{K}_{p}}\] is:
A)
\[0.46\]
done
clear
B)
\[0.61\]
done
clear
C)
\[0.95\]
done
clear
D)
\[0.73\]
done
clear
View Answer play_arrow
question_answer 72) The volume of a gas is reduced to \[1.0\,\,L\] at \[{{25}^{o}}C\] and \[1\,\,atm\] pressure. Its pressure at \[{{35}^{o}}C\] would be:
A)
\[0.96\,\,atm\]
done
clear
B)
\[1.03\,\,atm\]
done
clear
C)
\[2.04\,\,atm\]
done
clear
D)
\[3.08\,\,atm\]
done
clear
View Answer play_arrow
question_answer 73) The strongest acid among the following is:
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{2}}ClCOOH\]
done
clear
C)
\[C{{H}_{2}}FCOOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 74) The compressibility factor for an ideal gas is:
A)
\[<1\]
done
clear
B)
\[=1\]
done
clear
C)
\[>1\]
done
clear
D)
always\[2\]
done
clear
View Answer play_arrow
question_answer 75) The standard reduction potential of\[L{{i}^{+}},\,B{{a}^{2+}},\,\,N{{a}^{+}}\]and\[M{{g}^{2+}}\]are\[-3.05,\,\,-2.73,\]\[-2.71\] and \[-2.37\] volts respectively. Which one is the strongest reducing agent?
A)
\[Li\]
done
clear
B)
\[Na\]
done
clear
C)
\[Mg\]
done
clear
D)
\[Ba\]
done
clear
View Answer play_arrow
question_answer 76) The unit of rate constant for 1st order reaction is:
A)
\[mol\,\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
B)
\[mo{{l}^{-1}}L{{s}^{-1}}\]
done
clear
C)
\[{{s}^{-1}}\]
done
clear
D)
\[mo{{l}^{2}}{{L}^{-2}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 77) In which of the following, the dipole moment is zero?
A)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 78) The bond order of\[O_{2}^{+}\]is:
A)
\[2\]
done
clear
B)
\[2.5\]
done
clear
C)
\[1.5\]
done
clear
D)
\[0.5\]
done
clear
View Answer play_arrow
question_answer 79) Addition of \[HgC{{l}_{2}}\] to \[SnC{{l}_{2}}\] gives a black colour due to:
A)
oxidation of\[Sn\]
done
clear
B)
reduction of\[HgC{{l}_{2}}\]
done
clear
C)
formation of amalgam
done
clear
D)
oxidation of\[Hg\]
done
clear
View Answer play_arrow
question_answer 80) Which one of the following is a trihydric alcohol containing only secondary hydroxyl group?
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}OH\]
done
clear
B)
\[\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 81) How, many sigma and it-bonds are there in the following compound? \[C{{H}_{2}}=CH-C\equiv C-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\]
A)
\[11\sigma ,\,\,4\pi \]
done
clear
B)
\[12\sigma ,\,\,3\pi \]
done
clear
C)
\[14\sigma ,\,\,4\pi \]
done
clear
D)
\[15\sigma ,\,\,4\pi \]
done
clear
View Answer play_arrow
question_answer 82) In the nitration of benzene, the reactive species is:
A)
\[N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{+}\]
done
clear
C)
\[NO_{2}^{-}\]
done
clear
D)
\[N{{O}^{-}}\]
done
clear
View Answer play_arrow
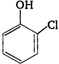
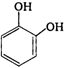
question_answer 83) Reaction of phenol with\[CC{{l}_{4}}\]and\[NaOH\], followed by hydrolysis is likely to give:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 84) In the reaction, \[_{92}{{U}^{238}}{{\xrightarrow{{}}}_{82}}P{{b}^{206}}\], the number of \[\alpha \] and \[\beta -\]particles emitted are:
A)
\[7\alpha ,\,\,5\beta \]
done
clear
B)
\[6\alpha ,\,\,4\beta \]
done
clear
C)
\[4\alpha ,\,\,3\beta \]
done
clear
D)
\[8\alpha ,\,\,6\beta \]
done
clear
View Answer play_arrow
question_answer 85) Which is the correct Gibb's Helmholtz equation?
A)
\[\Delta H=\Delta G=T\cdot \Delta S\]
done
clear
B)
\[\Delta S=\frac{1}{T}[\Delta G-\Delta H]\]
done
clear
C)
\[\Delta S=\frac{1}{T}[\Delta H-\Delta G]\]
done
clear
D)
\[-\Delta G=\Delta H-T\cdot \Delta S\]
done
clear
View Answer play_arrow
question_answer 86) Vinegar is:
A)
\[8-10%\] acetic acid
done
clear
B)
\[6-10%\] ethyl alcohol'
done
clear
C)
glacial acetic acid
done
clear
D)
\[10%\] formic acid
done
clear
View Answer play_arrow
question_answer 87) Decreasing order of electron affinity of halogens is:
A)
\[Cl>Br>F>I\]
done
clear
B)
\[Cl>F>Br>I\]
done
clear
C)
\[Br>Cl>F>I\]
done
clear
D)
\[F>Cl>Br>I\]
done
clear
View Answer play_arrow
question_answer 88) Which of the following is an acidic salt?
A)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
B)
\[Pb(OH)Cl\]
done
clear
C)
\[BaC{{l}_{2}}\]
done
clear
D)
\[N{{a}_{2}}HP{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 89) Which one is the Lewis acid?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[RN{{H}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 90) \[720\,\,g\] water contain the number of moles:
A)
\[2\]
done
clear
B)
\[190\]
done
clear
C)
\[40\]
done
clear
D)
\[55\]
done
clear
View Answer play_arrow
question_answer 91) \[2HCHO\xrightarrow{50%NaOH}C{{H}_{3}}OH+HCOONa\] This reaction is called:
A)
aldol condensation
done
clear
B)
Tischenko reaction
done
clear
C)
Cannizaro reaction
done
clear
D)
Reimer Tiemann reaction
done
clear
View Answer play_arrow
question_answer 92) The geometry of the molecule having \[s\] and \[p-\]characters equal to \[25%\] and \[75%\] respectively, is:
A)
trigonal
done
clear
B)
linear
done
clear
C)
tetrahedral
done
clear
D)
octahedral
done
clear
View Answer play_arrow
question_answer 93) Impossible configuration is:
A)
\[1{{s}^{2}},\,\,2{{s}^{2}}2{{p}^{2}},\,\,3{{s}^{2}}\]
done
clear
B)
\[1{{s}^{2}},\,\,2{{s}^{2}}\]
done
clear
C)
\[1{{s}^{2}},\,\,2{{s}^{2}}2{{p}^{6}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 94) The solubility product of \[PbB{{r}_{2}}\] is \[10.8\times {{10}^{-5}}\]. It, is \[70%\] dissociated in saturated solution. The solubility of salt is:
A)
\[4.18\times {{10}^{-2}}\]
done
clear
B)
\[6.76\times {{10}^{-3}}\]
done
clear
C)
\[3.4\times {{10}^{-4}}\]
done
clear
D)
\[5.44\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 95) The latent heat of vaporisation of water is \[540\,\,cal\,\,{{g}^{-1}}\] at \[{{100}^{o}}C\]. What will be the entropy increase when one mole of water is evaporated at\[{{100}^{o}}C?\]
A)
\[28\,\,cal\,\,{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
B)
\[26\,\,cal\,\,{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
C)
\[540\,\,cal\,\,{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
D)
\[1.82\,\,cal\,\,{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 96) The brown ring in the test of nitrate is formed due to:
A)
\[{{[Fe{{({{H}_{2}}O)}_{5}}N{{O}_{2}}]}^{2+}}\]
done
clear
B)
\[Fe{{(N{{O}_{3}})}_{3}}\]
done
clear
C)
\[{{[Fe{{({{H}_{2}}O)}_{5}}NO]}^{2+}}\]
done
clear
D)
\[{{[Fe({{H}_{2}}O){{(NO)}_{5}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 97) The corrosive sublimate is :
A)
\[Hg\]
done
clear
B)
\[H{{g}_{2}}O\]
done
clear
C)
\[H{{g}_{2}}C{{l}_{2}}\]
done
clear
D)
\[HgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 98) The half-life of radium is\[1580\,\,yr\]. Its average life is:
A)
\[2.275\times {{10}^{3}}yr\]
done
clear
B)
\[3.735\times {{10}^{2}}yr\]
done
clear
C)
\[1.62\times {{10}^{3}}yr\]
done
clear
D)
\[7.28\times {{10}^{2}}yr\]
done
clear
View Answer play_arrow
question_answer 99) Cobalt is present in:
A)
vitamin\[{{B}_{12}}\]
done
clear
B)
vitamin\[{{B}_{6}}\]
done
clear
C)
vitamin\[{{B}_{2}}\]
done
clear
D)
vitamin\[{{B}_{1}}\]
done
clear
View Answer play_arrow
question_answer 100) Adipic acid and hexamethylene diamine, on polymerisation gives:
A)
nylon\[-6\]
done
clear
B)
Dacron
done
clear
C)
nylon\[-66\]
done
clear
D)
bakelite
done
clear
View Answer play_arrow
question_answer 101) If \[z=x+iy\], then the area of a triangle whose vertices are points \[z,\,\,iz\] and \[z+iz\] is:
A)
\[2|z{{|}^{2}}\]
done
clear
B)
\[\frac{1}{2}|z{{|}^{2}}\]
done
clear
C)
\[|z{{|}^{2}}\]
done
clear
D)
\[\frac{3}{2}|z{{|}^{2}}\]
done
clear
View Answer play_arrow
question_answer 102) If the-complex number \[{{z}_{1}},\,\,{{z}_{2}}\] and \[{{z}_{3}}\] represent the vertices of an equilateral triangle such that \[|{{z}_{1}}|=|{{z}_{2}}|=|{{z}_{3}}|\], then \[{{z}_{1}}+{{z}_{2}}+{{z}_{3}}\] is equal to:
A)
\[0\]
done
clear
B)
\[1\]
done
clear
C)
\[-1\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 103) If\[{{i}^{2}}=-1\], then the value of\[\sum\limits_{n=1}^{200}{{{i}^{n}}}\]is:
A)
\[50\]
done
clear
B)
\[-50\]
done
clear
C)
\[0\]
done
clear
D)
\[100\]
done
clear
View Answer play_arrow
question_answer 104) The sum to infinity of the series\[2+\frac{1}{2}+\frac{1}{3}+\frac{1}{{{2}^{2}}}+\frac{1}{{{3}^{2}}}+\frac{1}{{{2}^{3}}}+\frac{1}{{{3}^{3}}}+...\]
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[\frac{7}{2}\]
done
clear
D)
\[\frac{9}{2}\]
done
clear
View Answer play_arrow
question_answer 105) If\[x>1,\,\,y>1,\,\,z>1\]are in\[GP\], then\[\frac{1}{1+\log x},\,\,\frac{1}{1+\log y},\,\,\frac{1}{1+\log z}\]are in:
A)
\[AP\]
done
clear
B)
\[HP\]
done
clear
C)
\[GP\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 106) If\[{{x}^{2}}-3x+2\]be a factor of\[{{x}^{4}}-p{{x}^{2}}+q\], then \[(p,\,\,q)\]is equal to:
A)
\[(3,\,\,4)\]
done
clear
B)
\[(4,\,\,5)\]
done
clear
C)
\[(4,\,\,3)\]
done
clear
D)
\[(5,\,\,4)\]
done
clear
View Answer play_arrow
question_answer 107) If\[a{{x}^{2}}+bx+c=0\]and\[b{{x}^{2}}+cx+a=0\], then a common root and\[a\ne 0\], then\[\frac{{{a}^{3}}+{{b}^{3}}+{{c}^{3}}}{abc}\]is equal to:
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 108) If\[^{15}{{C}_{3r}}{{=}^{15}}{{C}_{r+3}}\], then the value of\[r\]is:
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[5\]
done
clear
D)
\[8\]
done
clear
View Answer play_arrow
question_answer 109) The number of divisors of \[9600\] including \[1\] and \[9600\] are:
A)
\[60\]
done
clear
B)
\[58\]
done
clear
C)
\[48\]
done
clear
D)
\[46\]
done
clear
View Answer play_arrow
question_answer 110) If\[{{a}_{n}}=\sum\limits_{r=0}^{n}{\frac{1}{^{n}{{C}_{r}}}}\], then\[\sum\limits_{r=0}^{n}{\frac{r}{^{n}{{C}_{r}}}}\]is equal to:
A)
\[(n-1){{a}_{n}}\]
done
clear
B)
\[n{{a}_{n}}\]
done
clear
C)
\[\frac{1}{2}n{{a}_{n}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 111) 6th term in expansion of\[{{\left( 2{{x}^{2}}-\frac{1}{3{{x}^{2}}} \right)}^{10}}\]is:
A)
\[\frac{4580}{17}\]
done
clear
B)
\[-\frac{896}{27}\]
done
clear
C)
\[\frac{5580}{17}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 112) In the expansion of\[{{(1+x)}^{m}}{{(1-x)}^{n}}\], the coefficient of \[x\] and\[{{x}^{2}}\]are \[3\] and \[-6\] respectively, then \[m\] is equal to:
A)
\[6\]
done
clear
B)
\[9\]
done
clear
C)
\[12\]
done
clear
D)
\[24\]
done
clear
View Answer play_arrow
question_answer 113) \[(1+3){{\log }_{e}}3+\frac{1+{{3}^{2}}}{2!}{{({{\log }_{e}}3)}^{2}}\]\[+\frac{1+{{3}^{3}}}{3!}{{({{\log }_{e}}3)}^{3}}+...\infty \]is equal to:
A)
\[28\]
done
clear
B)
\[30\]
done
clear
C)
\[25\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 114) The sum of\[\frac{1}{2}+\frac{1}{3}+\frac{1}{{{2}^{3}}}+\frac{1}{5}\cdot \frac{1}{{{2}^{5}}}+...\infty \]is:
A)
\[{{\log }_{e}}\sqrt{\frac{3}{2}}\]
done
clear
B)
\[{{\log }_{e}}\sqrt{3}\]
done
clear
C)
\[{{\log }_{e}}\sqrt{\frac{7}{2}}\]
done
clear
D)
\[{{\log }_{e}}3\]
done
clear
View Answer play_arrow
question_answer 115) \[\left| \begin{matrix} 1 & a & {{a}^{2}}-bc \\ 1 & b & {{b}^{2}}-ac \\ 1 & c & {{c}^{2}}-ac \\ \end{matrix} \right|\]is equal to:
A)
\[0\]
done
clear
B)
\[{{a}^{3}}+{{b}^{3}}+{{c}^{3}}-3abc\]
done
clear
C)
\[3abc\]
done
clear
D)
\[{{(a+b+c)}^{3}}\]
done
clear
View Answer play_arrow
question_answer 116) \[x+ky-z=0\],\[3x-ky-z=0\]and\[x-3y+z=0\] has non-zero solution for \[k\] is equal to:
A)
\[-1\]
done
clear
B)
\[0\]
done
clear
C)
\[1\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 117) If\[A=\left| \begin{matrix} 1 & -2 & 3 \\ 0 & -1 & 4 \\ -2 & 2 & 1 \\ \end{matrix} \right|\], then\[{{(A')}^{-1}}\] is equal to:
A)
\[\left[ \begin{matrix} -9 & -8 & -2 \\ 8 & 7 & 2 \\ -5 & -4 & -1 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 1 & -1 & -2 \\ -2 & -1 & 2 \\ 3 & 4 & 1 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} -9 & 8 & 5 \\ -8 & 7 & -4 \\ 2 & 2 & -1 \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} 1 & 0 & 0 \\ 0 & 1 & 0 \\ 0 & 0 & 1 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 118) Inverse matrix of\[\left[ \begin{matrix} 4 & 7 \\ 1 & 2 \\ \end{matrix} \right]\]is equal to:
A)
\[\left[ \begin{matrix} 2 & -7 \\ -1 & 4 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 2 & -1 \\ -7 & 4 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} -2 & 7 \\ 1 & -4 \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} -2 & 1 \\ 7 & -4 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 119) If \[A\] lies in the second quadrant and\[3\tan A+4=0\], then value of\[2\cot A-5\cos A+\sin A\]is equal to:
A)
\[-53/10\]
done
clear
B)
\[-7/10\]
done
clear
C)
\[7/10\]
done
clear
D)
\[23/10\]
done
clear
View Answer play_arrow
question_answer 120) If\[\sin \theta +\cos \theta =1\], then the general value of\[\theta \]is:
A)
\[2n\pi \]
done
clear
B)
\[n\pi +{{(-1)}^{n}}\frac{\pi }{4}-\frac{\pi }{4}\]
done
clear
C)
\[2n\pi +\frac{\pi }{2}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 121) \[\cos \left[ 2{{\cos }^{-1}}\frac{1}{5}+{{\sin }^{-1}}\frac{1}{5} \right]\]is equal to:
A)
\[\frac{2\sqrt{6}}{5}\]
done
clear
B)
\[\frac{-2\sqrt{6}}{5}\]
done
clear
C)
\[\frac{1}{5}\]
done
clear
D)
\[\frac{-1}{5}\]
done
clear
View Answer play_arrow
question_answer 122) From a roof of a \[15\,\,m\] high house the angle of elevation of a point located \[15\,\,m\] distant to the base of the house is:
A)
\[{{45}^{o}}\]
done
clear
B)
\[{{30}^{o}}\]
done
clear
C)
\[{{60}^{o}}\]
done
clear
D)
\[{{90}^{o}}\]
done
clear
View Answer play_arrow
question_answer 123) If \[A\] and \[B\] are two fixed point and \[P\] is a variable point such that\[PA+PB=4\], then the locus of \[P\] is a/an:
A)
parabola
done
clear
B)
ellipse
done
clear
C)
hyperbola
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 124) A line passes through \[(2,\,\,2)\] and is perpendicular to the line \[3x+y=3\], its \[y-\]intercept is:
A)
\[1/3\]
done
clear
B)
\[2/3\]
done
clear
C)
\[1\]
done
clear
D)
\[4/3\]
done
clear
View Answer play_arrow
question_answer 125) The angle between the pair of straight lines \[{{x}^{2}}-{{y}^{2}}-2xy-1=0\]is :
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{75}^{o}}\]
done
clear
D)
\[{{36}^{o}}\]
done
clear
View Answer play_arrow
question_answer 126) The point of intersection of the line \[4x-3y-10=0\] and the circle \[{{x}^{2}}+{{y}^{2}}-2x+4y-20=0\] are:
A)
\[(-2,\,\,-6),\,\,(4,\,\,2)\]
done
clear
B)
\[(2,\,\,6),\,\,(-4,\,\,-2)\]
done
clear
C)
\[(-2,\,\,6),\,\,(-4,\,\,2)\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 127) The focus of the parabola\[{{y}^{2}}=4y-4x\]is:
A)
\[(0,\,\,2)\]
done
clear
B)
\[(1,\,\,2)\]
done
clear
C)
\[(2,\,\,0)\]
done
clear
D)
\[(2,\,\,1)\]
done
clear
View Answer play_arrow
question_answer 128) The eccentricity of the ellipse\[4{{x}^{2}}+9{{y}^{2}}+8x+36y+4=0\]is:
A)
\[5/6\]
done
clear
B)
\[3/5\]
done
clear
C)
\[\sqrt{2}/3\]
done
clear
D)
\[\sqrt{5}/3\]
done
clear
View Answer play_arrow
question_answer 129) The angle of intersection of the curves\[{{y}^{2}}=2x/\pi \]and\[y=\sin x\]is:
A)
\[{{\cot }^{-1}}(-1/\pi )\]
done
clear
B)
\[{{\cot }^{-1}}\pi \]
done
clear
C)
\[{{\tan }^{-1}}(-\pi )\]
done
clear
D)
\[{{\cot }^{-1}}\left( \frac{1}{\pi } \right)\]
done
clear
View Answer play_arrow
question_answer 130) The projection of any line on coordinate axes be respectively \[3,\,\,\,4,\,\,\,5,\] then its length is:
A)
\[12\]
done
clear
B)
\[50\]
done
clear
C)
\[5\sqrt{2}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 131) If the direction ratios of a line are \[1,\,\,-3,\,\,2,\] then the direction cosines of the line are :
A)
\[\frac{1}{\sqrt{14}},\,\,\frac{-3}{\sqrt{14}},\,\,\frac{2}{\sqrt{14}}\]
done
clear
B)
\[\frac{1}{\sqrt{14}},\,\,\frac{2}{\sqrt{14}},\,\,\frac{3}{\sqrt{14}}\]
done
clear
C)
\[\frac{-1}{\sqrt{14}},\,\,\frac{3}{\sqrt{14}},\,\frac{-2}{\sqrt{14}}\]
done
clear
D)
\[\frac{-1}{\sqrt{14}},\,\,\frac{-2}{\sqrt{14}},\,\,\frac{-3}{\sqrt{14}}\]
done
clear
View Answer play_arrow
question_answer 132) The area of triangle whose vertices are\[A(1,\,\,-1,\,\,2),\,\,\,B(2,\,\,1,\,\,-1)\]and\[C(3,\,\,-1,\,\,2)\]is:
A)
\[13\,\,sq\,\,unit\]
done
clear
B)
\[\sqrt{13}\,\,sq\,\,unit\]
done
clear
C)
\[56\,\,sq\,\,unit\]
done
clear
D)
\[\sqrt{6}\,\,sq\,\,unit\]
done
clear
View Answer play_arrow
question_answer 133) \[|(\overset{\to }{\mathop{\mathbf{a}}}\,\times \overset{\to }{\mathop{\mathbf{b}}}\,)\cdot \overset{\to }{\mathop{\mathbf{c}}}\,|=|\overset{\to }{\mathop{\mathbf{a}}}\,||\overset{\to }{\mathop{\mathbf{b}}}\,||\overset{\to }{\mathop{\mathbf{c}}}\,|\]if:
A)
\[\overset{\to }{\mathop{\mathbf{a}}}\,\cdot \overset{\to }{\mathop{\mathbf{b}}}\,=\overset{\to }{\mathop{\mathbf{b}}}\,\cdot \overset{\to }{\mathop{\mathbf{c}}}\,=0\]
done
clear
B)
\[\overset{\to }{\mathop{\mathbf{b}}}\,\cdot \overset{\to }{\mathop{\mathbf{c}}}\,=\overset{\to }{\mathop{\mathbf{c}}}\,\cdot \overset{\to }{\mathop{\mathbf{a}}}\,=0\]
done
clear
C)
\[\overset{\to }{\mathop{\mathbf{c}}}\,\cdot \overset{\to }{\mathop{\mathbf{a}}}\,=\overset{\to }{\mathop{\mathbf{a}}}\,\cdot \overset{\to }{\mathop{\mathbf{b}}}\,=0\]
done
clear
D)
\[\overset{\to }{\mathop{\mathbf{a}}}\,\cdot \overset{\to }{\mathop{\mathbf{b}}}\,=\overset{\to }{\mathop{\mathbf{b}}}\,\cdot \overset{\to }{\mathop{\mathbf{c}}}\,=\overset{\to }{\mathop{\mathbf{c}}}\,\cdot \overset{\to }{\mathop{\mathbf{a}}}\,=0\]
done
clear
View Answer play_arrow
question_answer 134) The angle between the vectors \[(2\widehat{\mathbf{i}}+6\widehat{\mathbf{j}}+3\widehat{\mathbf{k}})\] and\[(12\widehat{\mathbf{i}}-4\widehat{\mathbf{j}}+3\widehat{\mathbf{k}})\]is:
A)
\[{{\cos }^{-1}}\left( \frac{1}{10} \right)\]
done
clear
B)
\[{{\cos }^{-1}}\left( \frac{9}{11} \right)\]
done
clear
C)
\[{{\cos }^{-1}}\left( \frac{9}{91} \right)\]
done
clear
D)
\[{{\cos }^{-1}}\left( \frac{1}{9} \right)\]
done
clear
View Answer play_arrow
question_answer 135) Domain of the function\[\sin \log \left( \frac{\sqrt{4-{{x}^{2}}}}{1-x} \right)\]is:
A)
\[[-2,\,\,1]\]
done
clear
B)
\[(-2,\,\,1)\]
done
clear
C)
\[[-2,\,\,1)\]
done
clear
D)
\[(-2,\,\,1]\]
done
clear
View Answer play_arrow
question_answer 136) If\[f(9)=9,\,\,f'(9)=4\]and\[\underset{x\to 9}{\mathop{\lim }}\,\frac{f(x)-9}{x-9}=4\]then \[\underset{x\to 9}{\mathop{\lim }}\,\frac{\sqrt{f(x)}-3}{\sqrt{x}-3}\]is equal to:
A)
\[2\]
done
clear
B)
\[4\]
done
clear
C)
\[-2\]
done
clear
D)
\[-4\]
done
clear
View Answer play_arrow
question_answer 137) Let \[f(x)\] be defined for all \[x>0\] and be continuous, let \[f(x)\] satisfy\[f\left( \frac{x}{y} \right)=f(x)-f(y)\]for all \[x,\,\,y,\] then:
A)
\[f(x)=\log x\]
done
clear
B)
\[f(x)\]is bounded
done
clear
C)
\[f\left( \frac{1}{2} \right)\to 0\]as\[x\to 0\]
done
clear
D)
\[x\,\,f(x)\to 1\]as\[x\to 0\]
done
clear
View Answer play_arrow
question_answer 138) The domain of the function\[y=\frac{1}{\sqrt{|x|-x}}\]is:
A)
\[(-\infty ,\,\,0)\]
done
clear
B)
\[(+\infty ,\,\,0)\]
done
clear
C)
\[(-\infty ,\,\,-1)\]
done
clear
D)
\[(-\infty ,\,\,\infty )\]
done
clear
View Answer play_arrow
question_answer 139) Differential coefficient of \[{{\sin }^{-1}}\frac{1-x}{1+x}\] with respect to\[\sqrt{x}\]is:
A)
\[\frac{1}{2\sqrt{x}}\]
done
clear
B)
\[\frac{\sqrt{x}}{\sqrt{1-x}}\]
done
clear
C)
\[1\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 140) The number that exceeds its square by the greatest number is:
A)
\[-1\]
done
clear
B)
\[0\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 141) \[\int{\frac{{{\sin }^{8}}x-{{\cos }^{8}}x}{1-2{{\sin }^{2}}x{{\cos }^{2}}x}dx}\]is equal to:
A)
\[\sin 2x+c\]
done
clear
B)
\[-\frac{1}{2}\sin 2x+c\]
done
clear
C)
\[\frac{1}{2}\sin 2x+c\]
done
clear
D)
\[-\sin 2x+c\]
done
clear
View Answer play_arrow
question_answer 142) If\[f(x)=A\sin \left( \frac{\pi x}{2} \right)+B,\,\,\,f'\left( \frac{1}{2} \right)=\sqrt{2}\]and\[\int_{0}^{1}{f(x)}dx=\frac{2A}{\pi }\], then the constants \[A\] and \[B\] are respectively:
A)
\[\frac{\pi }{2}\]and\[\frac{\pi }{2}\]
done
clear
B)
\[\frac{2}{\pi }\]and\[\frac{3}{\pi }\]
done
clear
C)
\[\frac{4}{\pi }\]and\[0\]
done
clear
D)
\[0\]and\[\frac{-4}{\pi }\]
done
clear
View Answer play_arrow
question_answer 143) Let\[f(x)=x-[x]\]for all real number, where\[[x]\]is the integral part of\[x\], then\[\int_{-1}^{1}{f(x)}dx\]is equal to:
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[0\]
done
clear
D)
\[1/2\]
done
clear
View Answer play_arrow
question_answer 144) A solution of the differential equation\[{{\left( \frac{dy}{dx} \right)}^{2}}-x\frac{dy}{dx}+y=0\]is:
A)
\[y=2\]
done
clear
B)
\[y=2x\]
done
clear
C)
\[y=2x-4\]
done
clear
D)
\[y=2{{x}^{2}}-4\]
done
clear
View Answer play_arrow
question_answer 145) If \[A\] and \[B\] are two independent events, then \[P(A+B)\] is equal to:
A)
\[P(A)+P(B)-P(A)P(B)\]
done
clear
B)
\[P(A)-P(B)\]
done
clear
C)
\[P(A)+P(B)\]
done
clear
D)
\[P(A)+P(B)+P(A)P(B)\]
done
clear
View Answer play_arrow
question_answer 146) A bag contains \[6\] red, \[5\] white and \[4\] black balls, two balls are drawn the probability that none of them is red, is:
A)
\[12/35\]
done
clear
B)
\[6/35\]
done
clear
C)
\[4/35\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 147) If\[P(A)=0.65,\,\,P(B)=0.15\], then\[P(\bar{A})+P(\bar{B})\]is equal to:
A)
\[1.5\]
done
clear
B)
\[1.2\]
done
clear
C)
\[0.8\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 148) If the regression equations of the variables \[x\]and \[y\] be \[x=19.13-0.83y\] and\[y=11.64-0.50x\], then the correlation coefficient between x and y is:
A)
\[0.66\]
done
clear
B)
\[-0.64\]
done
clear
C)
\[0.001\]
done
clear
D)
\[-0.001\]
done
clear
View Answer play_arrow
question_answer 149) The roots of \[x+{{\log }_{10}}x=3.375\] is approximately:
A)
\[2.09\]
done
clear
B)
\[2.909\]
done
clear
C)
\[2.990\]
done
clear
D)
\[3.003\]
done
clear
View Answer play_arrow
question_answer 150) After second iteration of Newton-Raphson method, the positive root of equation \[{{x}^{2}}=3\](taking initial approximation\[\frac{3}{2}):\]
A)
\[3/2\]
done
clear
B)
\[7/4\]
done
clear
C)
\[97/56\]
done
clear
D)
\[347/200\]
done
clear
View Answer play_arrow