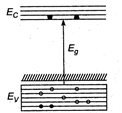
A) p-type semiconductor
B) insulator
C) metal
D) \[n-\]type semiconductor
Correct Answer: A
Solution :
The given figure represents p-type semiconductor as described below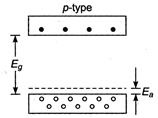 When one of the silicon atoms (valence = 4) has been replaced by an atom of aluminium (valence = 3), the aluminium atom can bond covalently with only three silicon atoms, so there is now a missing electron (a hole) in one aluminium-silicon bond with a small expanditure of energy, an electron can be torn from a neighbouring silicon-silicon bond to fill this hole, thereby creating a hole in that bond. Similarly, an electron from some other bond can be moved to fill the second hole. In this way, the hole can migrate through the lattice. The aluminium atom is called an acceptor atom because it readily accepts an electron from a neighbouring bond that is from the valence band of silicon. As figure suggests, this electron occupies a localized acceptor state that lies within the energy gap, at an average energy interval \[{{\text{E}}_{\text{a}}}\]above the top of the valence band. By adding acceptor atoms, it is possible to increase very greatly the number of holes in the valence band.
When one of the silicon atoms (valence = 4) has been replaced by an atom of aluminium (valence = 3), the aluminium atom can bond covalently with only three silicon atoms, so there is now a missing electron (a hole) in one aluminium-silicon bond with a small expanditure of energy, an electron can be torn from a neighbouring silicon-silicon bond to fill this hole, thereby creating a hole in that bond. Similarly, an electron from some other bond can be moved to fill the second hole. In this way, the hole can migrate through the lattice. The aluminium atom is called an acceptor atom because it readily accepts an electron from a neighbouring bond that is from the valence band of silicon. As figure suggests, this electron occupies a localized acceptor state that lies within the energy gap, at an average energy interval \[{{\text{E}}_{\text{a}}}\]above the top of the valence band. By adding acceptor atoms, it is possible to increase very greatly the number of holes in the valence band.
You need to login to perform this action.
You will be redirected in
3 sec
