A) trigonal bipyramidal geometry, linear shape
B) hexagonal geometry, T-shape
C) triangular planar geometry, triangular shape
D) tetrahedral geometry, pyramidal shape
Correct Answer: A
Solution :
\[I_{3}^{-}\Rightarrow 2\] 2 bond pairs + 3 lone pairs =5 hybrid orbitals. Thus, hybridisation is \[s{{p}^{3}}d\]and geometrical arrangement is trigonal bipyramidal. However, its shape is linear because of the presence of three lone pairs of electrons.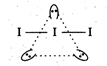
You need to login to perform this action.
You will be redirected in
3 sec
