-
question_answer1) Which one of the following compounds is an anti-fertility drug?
JEE Main Online Paper (Held On 07 May 2012)
A)
Aspirin
done
clear
B)
Chloro my cetin
done
clear
C)
Saheli
done
clear
D)
Penicillin
done
clear
View Answer play_arrow
-
question_answer2) The most basic compound among the following is
JEE Main Online Paper (Held On 07 May 2012)
A)
Acetanilide
done
clear
B)
Benzyl amine
done
clear
C)
p-Nitro aniline
done
clear
D)
Aniline
done
clear
View Answer play_arrow
-
question_answer3) A battery is constructed of Cr and \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}.\]The unbalanced chemical equation when such a battery discharges is following: \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}+Cr+{{H}^{+}}\to C{{r}^{3+}}+{{H}_{2}}O+N{{a}^{+}}\] If one Faraday of electricity is passed through the battery during the charging, the number of moles of \[C{{r}^{3+}}\] removed from the solution is
JEE Main Online Paper (Held On 07 May 2012)
A)
\[\frac{4}{3}\]
done
clear
B)
\[\frac{1}{3}\]
done
clear
C)
\[\frac{3}{3}\]
done
clear
D)
\[\frac{2}{3}\]
done
clear
View Answer play_arrow
-
question_answer4) The limiting line in Balmer series will have a frequency of (Rydberg constant, \[{{R}_{\infty }}=3.29\times {{10}^{15}}\]cycles/s)
JEE Main Online Paper (Held On 07 May 2012)
A)
\[8.22\times {{10}^{14}}{{s}^{-1}}\]
done
clear
B)
\[3.29\times {{10}^{15}}{{s}^{-1}}\]
done
clear
C)
\[3.65\times {{10}^{14}}{{s}^{-1}}\]
done
clear
D)
\[5.26\times {{10}^{13}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer5) Which of the following cannot be represented by resonance structures?
JEE Main Online Paper (Held On 07 May 2012)
A)
Dimethyl ether
done
clear
B)
Nitrate anion
done
clear
C)
Carboxylate anion
done
clear
D)
Toluene
done
clear
View Answer play_arrow
-
question_answer6) Which of the oxide groups among the following cannot be reduced by carbon?
A)
\[C{{u}_{2}}O,Sn{{O}_{2}}\]
done
clear
B)
\[CaO,{{K}_{2}}O\]
done
clear
C)
\[PbO,F{{e}_{2}}{{O}_{4}}\]
done
clear
D)
\[F{{e}_{2}}{{O}_{3}},ZnO\]
done
clear
View Answer play_arrow
-
question_answer7) Among the following, the species having the smallest bond is
JEE Main Online Paper (Held On 07 May 2012)
A)
\[N{{O}^{-}}\]
done
clear
B)
\[N{{O}^{+}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
NO
done
clear
View Answer play_arrow
-
question_answer8) Based on lattice energy and other considerations, which one of the following alkali metal chloride is expected to have the highest melting point?
JEE Main Online Paper (Held On 07 May 2012)
A)
NaCI
done
clear
B)
KCl
done
clear
C)
LiCI
done
clear
D)
RbCl
done
clear
View Answer play_arrow
-
question_answer9) The entropy of a sample of a certain substance increases by \[0.836\,J\,{{K}^{-1}}\] on adding reversibly0.3344 J of heat at constant temperature. The temperature of the sample is:
JEE Main Online Paper (Held On 07 May 2012)
A)
2.5K
done
clear
B)
0.3K
done
clear
C)
0.016K
done
clear
D)
0.4K
done
clear
View Answer play_arrow
-
question_answer10) The electron affinity of chlorine is 3.7 eV. 1 gram of chlorine is completely converted to \[C{{l}^{-1}}\] ion in a gaseous state. (1 eV = 23.06 kcal \[mo{{l}^{-1}}\]).Energy released in the process is
JEE Main Online Paper (Held On 07 May 2012)
A)
4.8kcal
done
clear
B)
7.2kcal
done
clear
C)
8.2 kcal
done
clear
D)
2.4 kcal
done
clear
View Answer play_arrow
-
question_answer11) Copper wire test for halogens is known as
JEE Main Online Paper (Held On 07 May 2012)
A)
Duma's Test
done
clear
B)
Beilstein' s Test
done
clear
C)
Liebig'sTest
done
clear
D)
Lassigne's Test
done
clear
View Answer play_arrow
-
question_answer12) For 1 mol of an ideal gas at a constant temperature T, the plot of (log P) against (log V) is a(P: Pressure, V\ Volume)
JEE Main Online Paper (Held On 07 May 2012)
A)
Straight line parallel to x-axis.
done
clear
B)
Straight line with a negative slope.
done
clear
C)
Curve starting at origin.
done
clear
D)
Straight line passing through origin.
done
clear
View Answer play_arrow
-
question_answer13) Which one of the following is a chain growth polymerisation?
JEE Main Online Paper (Held On 07 May 2012)
A)
Nucleic acid
done
clear
B)
Polystyrene
done
clear
C)
Protein
done
clear
D)
Starch
done
clear
View Answer play_arrow
-
question_answer14) The d-electron configurations of \[C{{r}^{2+}},M{{n}^{2+}},F{{e}^{2+}}\]and \[C{{o}^{2+}}\]are \[{{d}^{4}},{{d}^{5}},{{d}^{6}}\]and \[{{d}^{7}}\] respectively. Which one of the following will exhibit the lowest paramagnetic behaviour? (Atomic no. Cr =24, Mn = 25, Fe = 26, Co = 27).
JEE Main Online Paper (Held On 07 May 2012)
A)
\[{{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
C)
\[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
D)
\[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
-
question_answer15) If x is the mass of the gas adsorbed on mass m of the adsorbent at pressure p, Freundlic had sorption isotherm gives a straight line on plotting
JEE Main Online Paper (Held On 07 May 2012)
A)
\[x/m\,vs\,p\]
done
clear
B)
\[x/m\,vs1/\,p\]
done
clear
C)
\[\log x/m\,vs\,\log \,p\]
done
clear
D)
\[\log x/m\,\,vs\,p\]
done
clear
View Answer play_arrow
-
question_answer16) The solubility (in mol\[{{L}^{-1}}\]) of AgCl\[({{K}_{sp}}=1.0\times {{10}^{-10}})\] in a 0.1 M KC1 solution will be
JEE Main Online Paper (Held On 07 May 2012)
A)
\[1.0\times {{10}^{-9}}\]
done
clear
B)
\[1.0\times {{10}^{-10}}\]
done
clear
C)
\[1.0\times {{10}^{-5}}\]
done
clear
D)
\[1.0\times {{10}^{-11}}\]
done
clear
View Answer play_arrow
-
question_answer17) Among the following which is the best description of water in the solid phase?
JEE Main Online Paper (Held On 07 May 2012)
A)
Covalent solid
done
clear
B)
Molecular solid
done
clear
C)
Ionic solid
done
clear
D)
Network solid
done
clear
View Answer play_arrow
-
question_answer18) All of the following statements apply to proteins except
JEE Main Online Paper (Held On 07 May 2012)
A)
Proteins generally have no definite melting point
done
clear
B)
Proteins contain the grouping 'CONH'
done
clear
C)
Proteins have high molecular weight
done
clear
D)
Proteins can only contain the elements C, H, O and N.
done
clear
View Answer play_arrow
-
question_answer19) \[{{K}_{1}},{{K}_{2}}\]and\[{{K}_{3}}\] are the equilibrium constants of the following reactions (I), (II) and (III)respectively: (i)\[{{N}_{2}}+2{{O}_{2}}2N{{O}_{2}}\](ii)\[2N{{O}_{2}}{{N}_{2}}+2{{O}_{2}}\] (iii)\[N{{O}_{2}}\frac{1}{2}{{N}_{2}}+{{O}_{2}}\] The correct relation from the following is
JEE Main Online Paper (Held On 07 May 2012)
A)
\[{{K}_{1}}=\frac{1}{{{K}_{2}}}=\frac{1}{{{K}_{3}}}\]
done
clear
B)
\[{{K}_{1}}=\frac{1}{{{K}_{2}}}=\frac{1}{{{({{K}_{3}})}^{2}}}\]
done
clear
C)
\[{{K}_{1}}=\sqrt{{{K}_{2}}}={{K}_{3}}\]
done
clear
D)
\[{{K}_{1}}=\frac{1}{{{K}_{2}}}={{K}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer20) The concentrated sulphuric acid that is peddled commercial is \[95%{{H}_{2}}S{{O}_{4}}\] by weight. If the density of this commercial acid is 1.834 g\[c{{m}^{-3}},\] the molarity of this solution is
JEE Main Online Paper (Held On 07 May 2012)
A)
17.8 M
done
clear
B)
12.0 M
done
clear
C)
10.5M
done
clear
D)
15.7M
done
clear
View Answer play_arrow
-
question_answer21) A solid has a 'bcc' structure. If the distance of nearest approach between two atoms is 1.73A,the edge length of the cell is
JEE Main Online Paper (Held On 07 May 2012)
A)
314.20pm
done
clear
B)
1.41pm
done
clear
C)
200pm
done
clear
D)
216pm
done
clear
View Answer play_arrow
-
question_answer22) Reaction rate between two substance A and B is expressed as following:\[\text{rate}\,\text{=k }\!\![\!\!\text{ A}{{\text{ }\!\!]\!\!\text{ }}^{\text{n}}}{{\text{ }\!\![\!\!\text{ B }\!\!]\!\!\text{ }}^{\text{m}}}\] If the concentration of A is doubled and concentration of B is made half of initialcon centration, the ratio of the new rate to the earlier rate will be:
JEE Main Online Paper (Held On 07 May 2012)
A)
\[m+n\]
done
clear
B)
\[n-m\]
done
clear
C)
\[\frac{1}{{{2}^{\left(m+n \right)}}}\]
done
clear
D)
\[{{2}^{\left( n-m \right)}}\]
done
clear
View Answer play_arrow
-
question_answer23) How many cyclic structures are possible for\[{{C}_{4}}{{H}_{6}}?\]
JEE Main Online Paper (Held On 07 May 2012)
A)
3
done
clear
B)
5
done
clear
C)
6
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer24) Which is not the correct Statement? (At.nos.\[\text{Ce}=\text{58},\text{Lu}=\text{71},\text{La}=\text{57},\text{Yb}=\text{7}0\])
JEE Main Online Paper (Held On 07 May 2012)
A)
Colour of \[Y{{b}^{3+}}\] ion is pink.
done
clear
B)
\[L{{a}^{3+}}\]is diamagnetic.
done
clear
C)
\[C{{e}^{4+}}\] has\[{{f}^{0}}\] configuration.
done
clear
D)
\[L{{u}^{3+}}\]had\[{{f}^{14}}\] configuration.
done
clear
View Answer play_arrow
-
question_answer25) In which of the following arrangements, the sequence is not strictly according to the property written against it?
JEE Main Online Paper (Held On 07 May 2012)
A)
\[C{{O}_{2}}<Si{{O}_{2}}<Sn{{O}_{2}}<Pb{{O}_{2}}\]: increasing oxidising power
done
clear
B)
\[N{{H}_{3}}<P{{H}_{3}}<As{{H}_{3}}<Sb{{H}_{3}}\]: increasing basic strength
done
clear
C)
\[HF < HCl < HBr < HI\]: increasing acid strength
done
clear
D)
\[B<C<O<N\]: increasing first ionization enthalpy.
done
clear
View Answer play_arrow
-
question_answer26)
The IUPAC name of the compound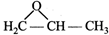 is
JEE Main Online Paper (Held On 07 May 2012)
is
JEE Main Online Paper (Held On 07 May 2012)
A)
1,2-Propoxide
done
clear
B)
Propylene oxide
done
clear
C)
1,2-Oxo propane
done
clear
D)
1,2-Epoxy propane
done
clear
View Answer play_arrow
-
question_answer27) Green house gases can be arranged in 'Global Warming Potential' sequence as
JEE Main Online Paper (Held On 07 May 2012)
A)
\[{{N}_{2}}O>CFC>C{{H}_{4}}>C{{O}_{2}}\]
done
clear
B)
\[CFC>{{N}_{2}}O>C{{H}_{4}}>C{{O}_{2}}\]
done
clear
C)
\[CFC>C{{O}_{2}}>{{N}_{2}}O>C{{H}_{4}}\]
done
clear
D)
\[C{{O}_{2}}>CFC>{{N}_{2}}O>C{{H}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer28) Among the following the order of reactivity towards nucieophilic addition is
JEE Main Online Paper (Held On 07 May 2012)
A)
\[C{{H}_{3}}CHO>C{{H}_{3}}COC{{H}_{3}}>HCHO\]
done
clear
B)
\[HCHO>C{{H}_{3}}CHO>C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}CHO>HCHO>C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}>C{{H}_{3}}CHO>HCHO\]
done
clear
View Answer play_arrow
-
question_answer29) \[{{C}_{2}}{{H}_{5}}Br\xrightarrow[{}]{AgCN}X\xrightarrow[Zn-Hg/HCl]{\operatorname{Re}duction}Y,\]Here Y is
JEE Main Online Paper (Held On 07 May 2012)
A)
Ethyl methyl amine
done
clear
B)
n-propylamine
done
clear
C)
Isopropylamine
done
clear
D)
Ethylamine
done
clear
View Answer play_arrow
-
question_answer30) The ratio of number of oxygen atoms (0) in 16.0 gozone \[({{O}_{3}}),\] 28.0 g carbon monoxide (CO) and16.0 oxygen \[({{O}_{2}})\] is (Atomic mass: C = 12,0 = 16and Avogadro's constant\[{{N}_{A}}=6.0\times {{10}^{23}}mo{{l}^{-1}}\])
JEE Main Online Paper (Held On 07 May 2012)
A)
3:1:2
done
clear
B)
1:1:2
done
clear
C)
3:1:1
done
clear
D)
1:1:1
done
clear
View Answer play_arrow
