-
question_answer1) Which one of the following properties is not shown by NO?
JEE Main Solved Paper-2014
A)
It combines with oxygen to form nitrogen dioxide
done
clear
B)
It is bond order is 2.5
done
clear
C)
It is diamagnetic in gaseous state
done
clear
D)
It is a neutral oxide
done
clear
View Answer play_arrow
-
question_answer2) If Z is a compressibility factor, van der Waals equation at low pressure can be written as :
JEE Main Solved Paper-2014
A)
\[Z=1-\frac{Pb}{RT}\]
done
clear
B)
\[Z=1+\frac{Pb}{RT}\]
done
clear
C)
\[Z=1+\frac{RT}{Pb}\]
done
clear
D)
\[Z=1-\frac{a}{VRT}\]
done
clear
View Answer play_arrow
-
question_answer3) 63. The metal that cannot be obtained by electrolysis of an aqueous solution of its salts is :
JEE Main Solved Paper-2014
A)
Cu
done
clear
B)
Cr
done
clear
C)
Ag
done
clear
D)
Ca
done
clear
View Answer play_arrow
-
question_answer4) Resistance of 0.2 M solution of an electrolyte is \[50\Omega .\]The specific conductance of the solution is\[\text{S}{{\text{m}}^{\text{-1}}}\]. The resistance of 0.5 M solution of the same electrolyte is \[280\Omega .\]The molarconductivity of 0.5 M solution of the electrolyte in \[\text{S}\,{{\text{m}}^{\text{2}}}\,\text{mo}{{\text{l}}^{-1}}\] is :
JEE Main Solved Paper-2014
A)
\[5\times {{10}^{3}}\]
done
clear
B)
\[5\times {{10}^{2}}\]
done
clear
C)
\[5\times {{10}^{-4}}\]
done
clear
D)
\[5\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
-
question_answer5) CsCl crystallises in body centred cubic lattice. If ?a? is its edge length then which of thefollowing expressions is correct ?
JEE Main Solved Paper-2014
A)
\[{{r}_{Cs}}+{{r}_{Cl-}}=\frac{\sqrt{3}}{2}a\]
done
clear
B)
\[{{r}_{Cs}}{{+}_{C{{l}^{-}}}}=\sqrt{3}a\]
done
clear
C)
\[{{r}_{Cs}}_{+}{{+}_{C{{l}^{-}}}}=3a\]
done
clear
D)
\[{{r}_{Cs}}_{+}+{{r}_{C{{l}^{-}}}}=\frac{3a}{2}\]
done
clear
View Answer play_arrow
-
question_answer6) Consider separate solutions of \[0.500M{{C}_{2}}{{H}_{5}}OH(aq),\] \[0.100M\,M{{g}_{3}}{{(P{{O}_{4}})}_{2}}(aq),0.250\,MKBr(aq)\]and \[0.125MN{{a}_{3}}P{{O}_{4}}(aq)\]at \[25{}^\circ C\]. Which statement is true about these solutions, assuming allsalts to be strong electrolytes ?
JEE Main Solved Paper-2014
A)
\[0.125MN{{a}_{3}}P{{O}_{4}}(aq)\]has the highest osmotic pressure.
done
clear
B)
\[0.500M{{C}_{2}}{{H}_{5}}OH(aq)\]has the highest osmotic pressure.
done
clear
C)
They all have the same osmotic pressure.
done
clear
D)
\[0.100MM{{g}_{3}}{{(P{{O}_{4}})}_{2}}(aq)\]has the highest osmotic pressure.
done
clear
View Answer play_arrow
-
question_answer7)
In which of the following reactions \[{{H}_{2}}{{O}_{2}}\]acts as a reducing agent ?
|
(A)\[{{H}_{2}}{{O}_{2}}+2{{H}^{+}}+2{{e}^{-}}\to 2{{H}_{2}}O\]
|
|
(B)\[{{H}_{2}}{{O}_{2}}-2{{e}^{-}}\to {{O}_{2}}+2{{H}^{+}}\]
|
|
(C)\[{{H}_{2}}{{O}_{2}}+2{{e}^{-}}\to 2O{{H}^{-}}\]
|
|
(D)\[{{H}_{2}}{{O}_{2}}+2O{{H}^{-}}-2{{e}^{-}}\to {{O}_{2}}+2{{H}_{2}}O\]
|
JEE Main Solved Paper-2014
A)
\[(a), (c)\]
done
clear
B)
\[(b), (d)\]
done
clear
C)
\[(a), (b)\]
done
clear
D)
\[(c), (d)\]
done
clear
View Answer play_arrow
-
question_answer8) In \[{{S}_{N}}2\]reactions, the correct order of reactivity for the following compounds : \[C{{H}_{3}}Cl,C{{H}_{3}}C{{H}_{2}}Cl,{{(C{{H}_{3}})}_{2}}CHCl\]and\[{{(C{{H}_{3}})}_{3}}CCl\]is:
JEE Main Solved Paper-2014
A)
\[C{{H}_{3}}C{{H}_{2}}Cl>C{{H}_{3}}Cl>{{(C{{H}_{3}})}_{2}}CHCl>{{(C{{H}_{3}})}_{3}}CCl\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CHCl>C{{H}_{3}}C{{H}_{2}}Cl>C{{H}_{3}}Cl>{{(C{{H}_{3}})}_{3}}CCl\]
done
clear
C)
\[C{{H}_{3}}Cl>{{(C{{H}_{3}})}_{2}}CHCl>C{{H}_{2}}C{{H}_{2}}Cl>{{(C{{H}_{3}})}_{3}}CCl\]
done
clear
D)
\[C{{H}_{3}}Cl>C{{H}_{3}}C{{H}_{2}}Cl>{{(C{{H}_{3}})}_{2}}CHCl>{{(C{{H}_{3}})}_{3}}CCl\]
done
clear
View Answer play_arrow
-
question_answer9) The octahedral complex of a metal ion \[{{M}^{3+}}\] with four monodentate ligands \[{{L}_{1}},{{L}_{2}},{{L}_{3}}\]and \[{{L}_{4}}\]absorb wavelengths in the region of red, green, yellow and blue, respectively. The increasingorder of ligand strength of the four ligands is :
JEE Main Solved Paper-2014
A)
\[{{L}_{3}}<{{L}_{2}}<{{L}_{4}}<{{L}_{1}}\]
done
clear
B)
\[{{L}_{1}}<{{L}_{2}}<{{L}_{4}}<{{L}_{3}}\]
done
clear
C)
\[{{L}_{4}}<{{L}_{3}}<{{L}_{2}}<{{L}_{1}}\]
done
clear
D)
\[{{L}_{1}}<{{L}_{3}}<{{L}_{2}}<{{L}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer10) For the estimation of nitrogen, 1.4 g of an organic compound was digested by Kjeldahl methodand the evolved ammonia was absorbed in 60 mL of\[\frac{M}{10}\]sulphuric acid. The unreacted acid required 20 mL of \[\frac{M}{10}\]sodium hydroxide for complete neutralization. The percentage of nitrogenin the compound is :
JEE Main Solved Paper-2014
A)
3 %
done
clear
B)
5 %
done
clear
C)
6 %
done
clear
D)
10 %
done
clear
View Answer play_arrow
-
question_answer11) The equivalent conductance of NaCl at concentration C and at infinite dilution are \[{{\lambda }_{C}}\] and \[{{\lambda }_{\infty }}\]respectively. The correct relationship between \[{{\lambda }_{C}}\] and \[{{\lambda }_{\infty }}\]is given as : (where the constant B is positive)
JEE Main Solved Paper-2014
A)
\[{{\lambda }_{C}}={{\lambda }_{\infty }}-(B)\sqrt{C}\]
done
clear
B)
\[{{\lambda }_{C}}={{\lambda }_{\infty }}+(B)\sqrt{C}\]
done
clear
C)
\[{{\lambda }_{C}}={{\lambda }_{\infty }}+(B)C\]
done
clear
D)
\[{{\lambda }_{C}}={{\lambda }_{\infty }}-(B)C\]
done
clear
View Answer play_arrow
-
question_answer12) For the reaction \[S{{O}_{2}}_{(g)}+\frac{1}{2}{{O}_{2(g)}}S{{O}_{3(g)}}\]if \[{{K}_{P}}={{K}_{C}}{{(RT)}^{x}}\] where the symbols have usual meaning then the value of x is : (assuming ideality)
JEE Main Solved Paper-2014
A)
\[\frac{1}{2}\]
done
clear
B)
1
done
clear
C)
-1
done
clear
D)
\[-\frac{1}{2}\]
done
clear
View Answer play_arrow
-
question_answer13) In the reaction, \[C{{H}_{3}}COOH\xrightarrow[{}]{LiAl{{H}_{4}}}A\xrightarrow[{}]{PC{{l}_{5}}}B\xrightarrow[{}]{Alc.KOH}C,\]the product C is :
JEE Main Solved Paper-2014
A)
Ethylene
done
clear
B)
Acetyl chloride
done
clear
C)
Acetaldehyde
done
clear
D)
Acetylene
done
clear
View Answer play_arrow
-
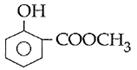
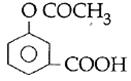
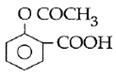
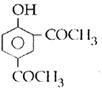
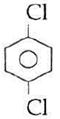
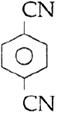
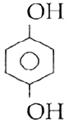
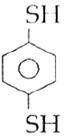
question_answer14)
Sodium phenoxide when heated with \[C{{O}_{2}}\]under pressure at \[125{}^\circ C\] yields a product which on acetylation produces C. 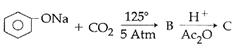 The major product C would be :
JEE Main Solved Paper-2014
The major product C would be :
JEE Main Solved Paper-2014
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer15) On heating an aliphatic primary amine with chloroform and ethanolic potassium hydroxide, theorganic compound formed is :
JEE Main Solved Paper-2014
A)
an alkyl cyanide
done
clear
B)
an alkyl isocyanide
done
clear
C)
analkanol
done
clear
D)
analkanediol
done
clear
View Answer play_arrow
-
question_answer16) The correct statement for the molecule, \[Cs{{I}_{3}},\]is :
JEE Main Solved Paper-2014
A)
it contains \[C{{s}^{3+}}\]and \[{{I}^{-}}\] ions.
done
clear
B)
it contains \[C{{s}^{+}},{{I}^{-}}\]and lattice \[{{I}_{2}}\] molecule.
done
clear
C)
it is a covalent molecule.
done
clear
D)
it contains \[C{{s}^{+}}\]and \[I_{3}^{-}\]ions.
done
clear
View Answer play_arrow
-
question_answer17) The equation which is balanced and represents the correct product(s) is :
JEE Main Solved Paper-2014
A)
\[{{[Mg{{({{H}_{2}}O)}_{6}}]}^{2+}}+{{(EDTA)}^{4-}}\xrightarrow[{}]{excessNaOH}{{[Mg(EDTA)]}^{2}}+6{{H}_{2}}O\]
done
clear
B)
\[CuS{{O}_{4}}+4KCN\to {{K}_{2}}[Cu{{(CN)}_{4}}]+{{K}_{2}}S{{O}_{4}}\]
done
clear
C)
\[L{{i}_{2}}+2KCl\to 2LiCl+{{K}_{2}}O\]
done
clear
D)
\[[CoCl{{(N{{H}_{3}})}_{5}}]++5{{H}^{+}}\to C{{o}^{2+}}+5NH_{4}^{+}+C{{l}^{-}}\]
done
clear
View Answer play_arrow
-
question_answer18)
For which of the following molecule significant \[\mu \ne 0?\]
JEE Main Solved Paper-2014
A)
\[Only (iii)\]
done
clear
B)
\[(iii) and (iv)\]
done
clear
C)
\[Only (i)\]
done
clear
D)
\[(i) and (ii)\]
done
clear
View Answer play_arrow
-
question_answer19)
For the non-stoichiometrereaction \[2A+B\to C+D,\]the following kinetic data were obtained inthree separate experiments, all at 298 K.
| Initial concentration (A) |
Initial Concentration (B) |
Initial rate of formation of C \[(mol\,{{L}^{-}}{{S}^{-}})\] |
| \[0.1M\] |
\[0.1M\] |
\[1.2\times {{10}^{-3}}\] |
| \[0.1M\] |
\[0.2M\] |
\[1.2\times {{10}^{-3}}\] |
| \[0.2M\] |
\[0.1M\] |
\[2.4\times {{10}^{-3}}\] |
The rate law for the formation of C is :
JEE Main Solved Paper-2014
A)
\[\frac{dc}{dt}=k[A]{{[B]}^{2}}\]
done
clear
B)
\[\frac{dc}{dt}=k[A]\]
done
clear
C)
\[\frac{dc}{dt}=k[A][B]\]
done
clear
D)
\[\frac{dc}{dt}=k{{[A]}^{2}}[B]\]
done
clear
View Answer play_arrow
-
question_answer20) Which series of reactions correctly represents chemical relations related to iron and itscompound?
JEE Main Solved Paper-2014
A)
\[Fe\xrightarrow[{}]{C{{l}_{2}}.heat}FeC{{l}_{3}}\xrightarrow[{}]{heat,air}FeC{{l}_{2}}\xrightarrow[{}]{Zn}Fe\]
done
clear
B)
\[Fe\xrightarrow[{}]{{{O}_{2}},heat}F{{e}_{3}}{{O}_{4}}\xrightarrow[{}]{CO,{{600}^{o}}C}FeO\xrightarrow[{}]{CO,{{700}^{o}}C}Fe\]
done
clear
C)
\[Fe\xrightarrow[{}]{dil\,{{H}_{2}}S{{O}_{4}}}FeS{{O}_{4}}\xrightarrow[{}]{{{H}_{2}}S{{O}_{4}},{{O}_{2}}}F{{e}_{2}}{{(S{{O}_{4}})}_{3}}\xrightarrow[{}]{heat}Fe\]
done
clear
D)
\[Fe\xrightarrow[{}]{{{O}_{2}},heat}FeO\xrightarrow[{}]{dil\,{{H}_{2}}S{{O}_{4}}}FeS{{O}_{4}}\xrightarrow[{}]{heat}Fe\]
done
clear
E)
done
clear
View Answer play_arrow
-
question_answer21) Considering the basic strength of amines in aqueous solution, which one has the smallest \[\text{p}{{\text{K}}_{\text{b}}}\]value?
JEE Main Solved Paper-2014
A)
\[{{(C{{H}_{3}})}_{3}}N\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
D)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer22) Which one of the following bases is not present in DNA?
JEE Main Solved Paper-2014
A)
Cytosine
done
clear
B)
Thymine
done
clear
C)
Quinoline
done
clear
D)
Adenine
done
clear
View Answer play_arrow
-
question_answer23) The correct set of four quantum numbers for the valence electrons of rubidium atom (Z = 37) is :
JEE Main Solved Paper-2014
A)
\[5,1,1,+\frac{1}{2}\]
done
clear
B)
\[5,0,1,+\frac{1}{2}\]
done
clear
C)
\[5,0,0,+\frac{1}{2}\]
done
clear
D)
\[5,1,0,+\frac{1}{2}\]
done
clear
View Answer play_arrow
-
question_answer24) The major organic compound formed by the reaction of 1, 1, 1 − trichloroethane with silver powder is :
JEE Main Solved Paper-2014
A)
2 - Butyne
done
clear
B)
2 - Butene
done
clear
C)
Acetylene
done
clear
D)
Ethene
done
clear
View Answer play_arrow
-
question_answer25) Given below are the half − cell reaction : \[M{{n}^{2+}}+2{{e}^{-}}\to Mn;{{E}^{o}}=-1.18V\] \[2(M{{n}^{3+}}+{{e}^{-}}\to M{{n}^{2+}});{{E}^{o}}=+1.51V.\] The \[E{}^\circ \]for \[3M{{n}^{2+}}\to Mn+2M{{n}^{3+}}\]will be
JEE Main Solved Paper-2014
A)
0.33 V ; the reaction will not occur
done
clear
B)
−0.33 V ; the reaction will occur
done
clear
C)
−2.69 V ; the reaction will not occur
done
clear
View Answer play_arrow
-
question_answer26) The ratio of masses of oxygen and nitrogen in a particular gaseous mixture is 1 : 4. The ratio ofnumber of their molecule is :
JEE Main Solved Paper-2014
A)
1 : 8
done
clear
B)
3 : 16
done
clear
C)
1 : 4
done
clear
D)
7 : 32
done
clear
View Answer play_arrow
-
question_answer27) Which one is classified as a condensation polymer?
JEE Main Solved Paper-2014
A)
Teflon
done
clear
B)
Acrylonitrile
done
clear
C)
Dacron
done
clear
D)
Neoprene
done
clear
View Answer play_arrow
-
question_answer28) Among the following oxoacids, the correct decreasing order of acid strength is :
JEE Main Solved Paper-2014
A)
\[HCl{{O}_{4}}>HCl{{O}_{3}}>HCl{{O}_{2}}>HOCl\]
done
clear
B)
\[HCl{{O}_{2}}>HCl{{O}_{4}}>HCl{{O}_{3}}>HOCl\]
done
clear
C)
\[HOCl>HCl{{O}_{2}}>HCl{{O}_{3}}>HCl{{O}_{4}}\]
done
clear
D)
\[HCl{{O}_{4}}>HOCl>HCl{{O}_{2}}>HCl{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer29) For complete combustion of ethanol, \[{{C}_{2}}{{H}_{5}}OH(\ell )+3{{O}_{2}}(g)\to 2C{{O}_{2}}(g)+3{{H}_{2}}O(\ell ),\]the amount ofheat produced as measured in bomb calorimeter, is \[1364.47\,\text{kJ}\,\text{mo}{{\text{l}}^{-1}}\] at \[25{}^\circ C\]. Assuming ideality theEnthalpy of combustion, \[\Delta \text{cH,}\] for the reaction will be : \[(\text{R = 8}\text{.314 kJ mo}{{\text{l}}^{\text{-1}}})\]
JEE Main Solved Paper-2014
A)
\[\text{-1460}\text{.50 kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
B)
\[\text{-1350}\text{.50 kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
C)
\[\text{-1366}\text{.95 kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
D)
\[\text{-1361}\text{.95 kJ mo}{{\text{l}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
-
question_answer30) The most suitable reagent for the conversion of \[R-C{{H}_{2}}-OH\to R-CHO\] is :
JEE Main Solved Paper-2014
A)
\[Cr{{O}_{3}}\]
done
clear
B)
PCC (PyridiniumChlorochromate)
done
clear
C)
\[KMn{{O}_{4}}\]
done
clear
D)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
View Answer play_arrow
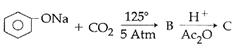 The major product C would be :
JEE Main Solved Paper-2014
The major product C would be :
JEE Main Solved Paper-2014