question_answer 1) Which of the following is a redox reaction?
AIEEE Solved Paper-2002
A)
\[NaCl+KN{{O}_{3}}\xrightarrow{{}}NaN{{O}_{3}}+KCl\]
done
clear
B)
\[Ca{{C}_{2}}{{O}_{4}}+2HCl\xrightarrow{{}}CaC{{l}_{2}}+{{H}_{2}}{{C}_{2}}{{O}_{4}}\]
done
clear
C)
\[Ca{{(OH)}_{2}}+2N{{H}_{4}}Cl\xrightarrow{{}}CaC{{l}_{2}}+2N{{H}_{3}}\]\[+2{{H}_{2}}O\]
done
clear
D)
\[2K[Ag{{(CN)}_{2}}]+Zn\xrightarrow{{}}2Ag+{{K}_{2}}[Zn{{(CN)}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 2) For an ideal gas, number of mol per litre in terms of its pressure p, temperature T and gas constant R is
AIEEE Solved Paper-2002
A)
\[\frac{pT}{R}\]
done
clear
B)
pRT
done
clear
C)
\[\frac{p}{RT}\]
done
clear
D)
\[\frac{RT}{p}\]
done
clear
View Answer play_arrow
question_answer 3) Number of P - O bonds in \[{{P}_{4}}{{O}_{10}}\] is
AIEEE Solved Paper-2002
A)
17
done
clear
B)
16
done
clear
C)
15
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 4) \[K{{O}_{2}}\] is used in space and submarines because it
AIEEE Solved Paper-2002
A)
absorbs \[C{{O}_{2}}\] and increases \[{{O}_{2}}\] concentration
done
clear
B)
absorbs moisture
done
clear
C)
absorbs \[C{{O}_{2}}\]
done
clear
D)
produces ozone
done
clear
View Answer play_arrow
question_answer 5) Which of the following ions has the maximum magnetic moment?
AIEEE Solved Paper-2002
A)
\[M{{n}^{2+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[T{{i}^{2+}}\]
done
clear
D)
\[C{{r}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 6) Acetylene does not react with
AIEEE Solved Paper-2002
A)
Na
done
clear
B)
ammoniacal \[AgN{{O}_{3}}\]
done
clear
C)
\[HCl\]
done
clear
D)
\[NaOH\]
done
clear
View Answer play_arrow
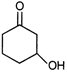
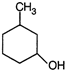
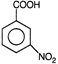
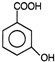
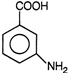
question_answer 7)
Compound A given below is
A)
antiseptic
done
clear
B)
antibiotic
done
clear
C)
analgesic
done
clear
D)
pesticide
done
clear
View Answer play_arrow
question_answer 8) For the following cell with hydrogen electrodes at two different pressures pi and?2 \[\underset{{{p}_{1}}}{\mathop{\operatorname{P}t({{H}_{2}})}}\,\,\,\underset{1M}{\mathop{\left| {{H}^{+}}(aq) \right|}}\,\,\,\underset{{{p}_{2}}}{\mathop{\operatorname{P}t({{H}_{2}})}}\,\] emf is given by
AIEEE Solved Paper-2002
A)
\[\frac{RT}{F}{{\log }_{e}}\frac{{{p}_{1}}}{{{p}_{2}}}\]
done
clear
B)
\[\frac{RT}{2F}{{\log }_{e}}\frac{{{p}_{1}}}{{{p}_{2}}}\]
done
clear
C)
\[\frac{RT}{F}{{\log }_{e}}\frac{{{p}_{2}}}{{{p}_{1}}}\]
done
clear
D)
\[\frac{RT}{2F}{{\log }_{e}}\frac{{{p}_{2}}}{{{p}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 9) Acetylene reacts with hypochlorous acid to form
AIEEE Solved Paper-2002
A)
\[C{{l}_{2}}CHCHO\]
done
clear
B)
\[ClC{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}COCl\]
done
clear
D)
\[ClC{{H}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 10) On heating benzyl amine with chloroform and ethanolic KOH, product obtained is
AIEEE Solved Paper-2002
A)
benzyl alcohol
done
clear
B)
benzaldehyde
done
clear
C)
benzonitrile
done
clear
D)
benzyl isocyanide
done
clear
View Answer play_arrow
question_answer 11) Which of the following reaction is possible at anode?
AIEEE Solved Paper-2002
A)
\[{{F}_{2}}+2{{e}^{-}}\xrightarrow{{}}2{{F}^{-}}\]
done
clear
B)
\[2{{H}^{+}}+\frac{1}{2}{{O}_{2}}+2{{e}^{-}}\xrightarrow{{}}{{H}_{2}}O\]
done
clear
C)
\[2C{{r}^{3+}}+7{{H}_{2}}O\xrightarrow{{}}C{{r}_{2}}O_{7}^{2-}+14{{H}^{+}}+6{{e}^{-}}\]
done
clear
D)
\[F{{e}^{2+}}\xrightarrow{{}}F{{e}^{3+}}+{{e}^{-}}\]
done
clear
View Answer play_arrow
question_answer 12) Which of the following concentration factor is affected by change in temperature?
AIEEE Solved Paper-2002
A)
Molarity
done
clear
B)
Molality
done
clear
C)
Mole fraction
done
clear
D)
Weight fraction
done
clear
View Answer play_arrow
question_answer 13) Cyanide process is used for the extraction of
AIEEE Solved Paper-2002
A)
barium
done
clear
B)
silver
done
clear
C)
boron
done
clear
D)
zinc
done
clear
View Answer play_arrow
question_answer 14) Following reaction, \[{{(C{{H}_{3}})}_{3}}CBr+{{H}_{2}}O\xrightarrow{{}}{{(C{{H}_{3}})}_{3}}COH+HBr\] is an example of
AIEEE Solved Paper-2002
A)
elimination reaction
done
clear
B)
free radical substitution
done
clear
C)
nucleophilic substitution
done
clear
D)
electrophilic substitution
done
clear
View Answer play_arrow
question_answer 15) A metal M forms water soluble \[MS{{O}_{4}}\] and inert MO. MO in aqueous solution forms insoluble \[M{{(OH)}_{2}}\] soluble in \[NaOH\]. Metal M is
AIEEE Solved Paper-2002
A)
\[Be\]
done
clear
B)
\[Mg\]
done
clear
C)
\[Ca\]
done
clear
D)
\[Si\]
done
clear
View Answer play_arrow
question_answer 16) Half-life of a substance A following first order kinetics is 5 days. Starting with 100g of A, amount left after 15 days is
AIEEE Solved Paper-2002
A)
25 g
done
clear
B)
50 g
done
clear
C)
\[12.5\]g
done
clear
D)
\[6.25\]g
done
clear
View Answer play_arrow
question_answer 17) The most stable ion is
AIEEE Solved Paper-2002
A)
\[{{[Fe{{(OH)}_{5}}]}^{3-}}\]
done
clear
B)
\[{{[FeC{{l}_{6}}]}^{3-}}\]
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
done
clear
D)
\[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 18) A substance forms Zwitter ion. It can have functional groups
AIEEE Solved Paper-2002
A)
\[-N{{H}_{2}},-COOH\]
done
clear
B)
\[-N{{H}_{2}},-S{{O}_{3}}H\]
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 19) If \[F{{e}^{3+}}\] and \[C{{r}^{3+}}\] both are present in group III of qualitative analysis, then distinction can be made by
AIEEE Solved Paper-2002
A)
addition of \[N{{H}_{4}}OH\] in the presence of \[N{{H}_{4}}Cl\] when only \[Fe{{(OH)}_{3}}\] is precipitated
done
clear
B)
addition of \[N{{H}_{4}}OH\]) in the presence of \[N{{H}_{4}}Cl\]I when \[Cr{{(OH)}_{3}}\] and \[Fe{{(OH)}_{3}}\] both are precipitated and on adding \[B{{r}_{2}}\] water and NaOH, \[Cr{{(OH)}_{3}}\] dissolves
done
clear
C)
precipitate of \[Cr{{(OH)}_{3}}\] and \[Fe{{(OH)}_{3}}\] as obtained in (b) are treated with cone. HCI when only \[Fe{{(OH)}_{3}}\] dissolves
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
question_answer 20) In an organic compound of molar mass \[108\,g\,mo{{l}^{-1}}\] C, H and N atoms are present in \[9:1:3.5\] by weight. Molecular formula can be
AIEEE Solved Paper-2002
A)
\[{{C}_{6}}{{H}_{8}}{{N}_{2}}\]
done
clear
B)
\[{{C}_{7}}{{H}_{10}}N\]
done
clear
C)
\[{{C}_{5}}{{H}_{6}}{{N}_{3}}\]
done
clear
D)
\[{{C}_{4}}{{H}_{18}}{{N}_{3}}\]
done
clear
View Answer play_arrow
question_answer 21) Solubility of \[Ca{{(OH)}_{2}}\] is s \[mol{{L}^{-1}}\]. The solubility product \[({{K}_{sp}})\] under the same condition is
AIEEE Solved Paper-2002
A)
\[4{{s}^{3}}\]
done
clear
B)
\[3{{s}^{4}}\]
done
clear
C)
\[4{{s}^{2}}\]
done
clear
D)
\[{{s}^{3}}\]
done
clear
View Answer play_arrow
question_answer 22) Heat required to raise the temperature of mole of a substance by \[{{1}^{o}}C\] is called
AIEEE Solved Paper-2002
A)
specific heat
done
clear
B)
molar heat capacity
done
clear
C)
water equivalent
done
clear
D)
specific gravity
done
clear
View Answer play_arrow
question_answer 23) \[\beta \]-particle is emitted in a radioactive reaction when
AIEEE Solved Paper-2002
A)
a proton changes to neutron
done
clear
B)
a neutron changes to proton
done
clear
C)
a neutron changes to electron
done
clear
D)
an electron changes to neutron
done
clear
View Answer play_arrow
question_answer 24) In a mixture of A and B, components show negative deviation when
AIEEE Solved Paper-2002
A)
A-B interaction is stronger than A-A and B-B interaction
done
clear
B)
A-B interaction is weaker than A-A and B-B interaction
done
clear
C)
\[\Delta {{V}_{\operatorname{mi}x}}>0\], \[\Delta {{S}_{\operatorname{mi}x}}>0\]
done
clear
D)
\[\Delta {{V}_{\operatorname{mi}x}}=0\], \[\Delta {{S}_{\operatorname{mi}x}}>0\]
done
clear
View Answer play_arrow
question_answer 25) Refining of impure copper with zinc impurity is to be done by electrolysis using electrodes as
AIEEE Solved Paper-2002
A)
Cathode - pure copper Anode- pure zinc
done
clear
B)
Cathode - pure zinc Anode- pure copper
done
clear
C)
Cathode - pure copper Anode- impure copper
done
clear
D)
Cathode - pure zinc Anode- impure zinc
done
clear
View Answer play_arrow
question_answer 26) Aluminium is extracted by the electrolysis of
AIEEE Solved Paper-2002
A)
alumina
done
clear
B)
bauxite
done
clear
C)
molten cryolite
done
clear
D)
alumina mixed with molten cryolite
done
clear
View Answer play_arrow
question_answer 27) For an aqueous solution, freezing point is\[{{0.186}^{o}}C\]. Elevation of the boiling point of the same solution is (\[{{K}_{f}}={{1.86}^{o}}mo{{l}^{-1}}kg\] and \[{{K}_{b}}={{0512}^{o}}mo{{l}^{-1}}kg\])
AIEEE Solved Paper-2002
A)
\[{{0.186}^{o}}\]
done
clear
B)
\[{{0.0.512}^{o}}\]
done
clear
C)
\[{{186}^{o}}\]
done
clear
D)
\[{{5.12}^{o}}\]
done
clear
View Answer play_arrow
question_answer 28) Underlined carbon is \[s{{p}^{3}}\] hybridised in
AIEEE Solved Paper-2002
A)
\[C{{H}_{3}}\underline{C}H=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}\underline{C}{{H}_{2}}N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}\underline{C}ON{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}\underline{C}N\]
done
clear
View Answer play_arrow
question_answer 29) Bond angle of \[{{109}^{o}}28\]'is found in
AIEEE Solved Paper-2002
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[\overset{\oplus }{\mathop{C}}\,{{H}_{5}}\]
done
clear
D)
\[\overset{\oplus }{\mathop{N}}\,{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 30) For a reaction \[A+2B\xrightarrow{{}}C\], rate is given by \[+\frac{d[C]}{dt}=k[A][B]\], hence the order of the reaction is
AIEEE Solved Paper-2002
A)
3
done
clear
B)
2
done
clear
C)
1
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 31) \[C{{H}_{3}}MgI\]is an organometallic compound due to
AIEEE Solved Paper-2002
A)
Mg-l bond
done
clear
B)
C-l bond
done
clear
C)
C-Mg bond
done
clear
D)
C-H bond
done
clear
View Answer play_arrow
question_answer 32) One of the following species acts as both Bronsted acid and base
AIEEE Solved Paper-2002
A)
\[{{H}_{2}}PO_{2}^{-}\]
done
clear
B)
\[HPO_{3}^{2-}\]
done
clear
C)
\[HPO_{4}^{2-}\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 33) Hybridisation of the underline atom changes in
AIEEE Solved Paper-2002
A)
\[\underline{A}\,l{{H}_{3}}\] changes to \[\underline{A}\,lH_{4}^{-}\]
done
clear
B)
\[{{H}_{2}}\underline{O}\] changes to \[{{H}_{3}}{{O}^{+}}\]
done
clear
C)
\[\underline{N}{{H}_{3}}\] changes to \[NH_{4}^{+}\]
done
clear
D)
in all the above cases
done
clear
View Answer play_arrow
question_answer 34) Racemic mixture is formed by mixing two
AIEEE Solved Paper-2002
A)
isomeric compounds
done
clear
B)
chiral compounds
done
clear
C)
meso compounds
done
clear
D)
enantiomers with chiral carbon
done
clear
View Answer play_arrow
question_answer 35) The number of lone pairs on \[Xe\] in \[Xe{{F}_{2}},Xe{{F}_{4}}\] and \[Xe{{F}_{6}}\]respectively are
AIEEE Solved Paper-2002
A)
3, 2, 1
done
clear
B)
2, 4, 6
done
clear
C)
1, 2, 3
done
clear
D)
6, 4, 2
done
clear
View Answer play_arrow
question_answer 36) An aqueous solution of \[1\,M\,NaCl\] and \[1\,M\,HCl\] is
AIEEE Solved Paper-2002
A)
not a buffer but \[pH<7\]
done
clear
B)
not a buffer but \[pH>7\]
done
clear
C)
a buffer with \[pH<7\]
done
clear
D)
a buffer with \[pH>7\]
done
clear
View Answer play_arrow
question_answer 37) Consider the following two reactions, \[A\xrightarrow{{}}\] Product \[-\frac{d[A]}{dt}={{k}_{1}}{{[A]}^{o}}\] \[B\xrightarrow{{}}\] Product \[-\frac{d[B]}{dt}={{k}_{2}}[B]\] \[{{k}_{1}}\] and \[{{k}_{2}}\] are expressed in terms of molarity(mol \[{{L}^{-1}}\]) and time \[({{s}^{-1}})\] as
AIEEE Solved Paper-2002
A)
\[{{s}^{-1}},M\,{{s}^{-1}}\,{{L}^{-1}}\]
done
clear
B)
\[M\,{{s}^{-1}},\,\,M\,{{s}^{-1}}\]
done
clear
C)
\[{{s}^{-1}},{{M}^{-1}}\,{{s}^{-1}}\]
done
clear
D)
\[M\,{{s}^{-1}},\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 38) RNA contains
A)
ribose sugar and thymine
done
clear
B)
ribose sugar and uracil
done
clear
C)
deoxyribose sugar and uracil
done
clear
D)
deoxyribose sugar and thymine
done
clear
View Answer play_arrow
question_answer 39) For a cell given below \[Ag\,\left| A{{g}^{+}} \right|\left| C{{u}^{2+}} \right|\,\,Cu\] - + \[A{{g}^{+}}+{{e}^{-}}\xrightarrow{{}}Ag,\,{{E}^{o}}=x\] \[C{{u}^{2+}}2\,{{e}^{-}}\xrightarrow{{}}Cu,\,{{E}^{o}}=y\] \[{{E}^{o}}_{cell}\] is
AIEEE Solved Paper-2002
A)
\[x+2y\]
done
clear
B)
\[2x+y\]
done
clear
C)
\[y-x\]
done
clear
D)
\[y-2x\]
done
clear
View Answer play_arrow
question_answer 40) Based on kinetic theory of gases, following laws can be proved
AIEEE Solved Paper-2002
A)
Boyle's law
done
clear
B)
Charle's law
done
clear
C)
Avogadro's law
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 41) \[MnO_{4}^{-}\] is a good oxidising agent in different medium changing to \[MnO_{4}^{-}\] \[\xrightarrow{{}}M{{n}^{2+}}\] \[\xrightarrow{{}}MnO_{4}^{2+}\] \[\xrightarrow{{}}Mn{{O}_{2}}\] \[\xrightarrow{{}}M{{n}_{2}}{{O}_{3}}\] Changes in oxidation number respectively are
AIEEE Solved Paper-2002
A)
1, 3, 4, 5
done
clear
B)
5, 4, 3, 2
done
clear
C)
5, 1, 3, 4
done
clear
D)
2, 6, 4, 3
done
clear
View Answer play_arrow
question_answer 42) For the reaction, \[{{H}_{2}}+{{I}_{2}}\xrightarrow{{}}2Hl\], the differential rate law is
AIEEE Solved Paper-2002
A)
\[-\frac{d\,[{{H}_{2}}]}{dt}=-\frac{d\,[{{l}_{2}}]}{dt}=2\frac{d\,[Hl]}{dt}\]
done
clear
B)
\[-2\frac{d\,[{{H}_{2}}]}{dt}=-2\frac{d\,[{{l}_{2}}]}{dt}=\frac{d\,[Hl]}{dt}\]
done
clear
C)
\[-\frac{d\,[{{H}_{2}}]}{dt}=-\frac{d\,[{{l}_{2}}]}{dt}=\frac{d\,[Hl]}{dt}\]
done
clear
D)
\[-\frac{d\,[{{H}_{2}}]}{2dt}=-\frac{d\,[{{l}_{2}}]}{2dt}=\frac{d\,[Hl]}{dt}\]
done
clear
View Answer play_arrow
question_answer 43) Number of atoms in 560 g of Fe (atomic mass 56 g \[mo{{l}^{-1}}\]) is
AIEEE Solved Paper-2002
A)
twice that of 70 g N
done
clear
B)
half that of 20 g H
done
clear
C)
Both (a) and (b)
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 44) Geometrical isomerism is not shown by
AIEEE Solved Paper-2002
A)
1, 1 -dichloro-1 ?pentene
done
clear
B)
1, 2-dichloro-l-pentene
done
clear
C)
1, 3-dichloro-2-pentene
done
clear
D)
1, 4-dichloro-2-pentene
done
clear
View Answer play_arrow
question_answer 45) Number of atoms in the unit cell of Na (bcc type crystal) and Mg (fee type crystal) are respectively
AIEEE Solved Paper-2002
A)
4, 4
done
clear
B)
4, 2
done
clear
C)
2, 4
done
clear
D)
1, 1
done
clear
View Answer play_arrow
question_answer 46) Which of the following compounds has incorrect IUPAC nomenclature?
AIEEE Solved Paper-2002
A)
\[C{{H}_{3}}\underset{\,\text{Ethylbutanoate}}{\mathop{C{{H}_{2}}\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}CO{{C}_{2}}}}\,{{H}_{5}}\]
done
clear
B)
\[C{{H}_{3}}CH\underset{\begin{smallmatrix} | \\ \underset{\text{3}-\text{ methyl butanal}}{\mathop{C{{H}_{3}}}}\, \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}CH\underset{\begin{smallmatrix} | \\ \underset{\text{2}-\text{methyl }-\text{3}-\text{ pentanone}}{\mathop{C{{H}_{3}}}}\, \end{smallmatrix}}{\mathop{\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,C}}\,{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[\underset{\text{2}-\text{ methyl }-\text{3}-\text{ butanol}}{\mathop{C{{H}_{3}}\underset{\begin{smallmatrix} | \\ {{H}_{3}}C \end{smallmatrix}}{\mathop{C}}\,H\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,HC{{H}_{3}}}}\,\]
done
clear
View Answer play_arrow
question_answer 47) End product of the following reaction is \[C{{H}_{3}}C{{H}_{2}}COOH\xrightarrow[\text{Red P}]{C\,{{l}_{2}}}\xrightarrow{\text{Alcoholic KOH}}\]
AIEEE Solved Paper-2002
A)
\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,HCOOH\]
done
clear
B)
\[C{{H}_{2}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{2}}=CHCOOH\]
done
clear
D)
\[\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,HCOOH\]
done
clear
View Answer play_arrow
question_answer 48) For the following reaction in gaseous phase \[CO+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}C{{O}_{2}}\] \[{{K}_{c}}/{{K}_{p}}\] is
AIEEE Solved Paper-2002
A)
\[{{(RT)}^{1/2}}\]
done
clear
B)
\[{{(RT)}^{-1/2}}\]
done
clear
C)
(RT)
done
clear
D)
\[{{(RT)}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 49) Energy of H-atom in the ground state is -13.6 eV, hence energy in the second excited state is
AIEEE Solved Paper-2002
A)
\[-6.8\] eV
done
clear
B)
\[-3.4\] eV
done
clear
C)
\[-1.51\] eV
done
clear
D)
\[-4.53\] Ev
done
clear
View Answer play_arrow
question_answer 50) A square planar complex is formed by hybridisation of the following atomic orbitals
AIEEE Solved Paper-2002
A)
\[s,{{p}_{x}},{{p}_{y}},{{p}_{Z}}\]
done
clear
B)
\[s,{{p}_{x}},{{p}_{y}},{{p}_{Z}},d\]
done
clear
C)
\[d,s,{{p}_{x}},{{p}_{y}}\]
done
clear
D)
\[s,{{p}_{x}},{{p}_{y}},{{p}_{Z}},d,d\]
done
clear
View Answer play_arrow
question_answer 51) Type of isomerism shown by \[[Cr{{(N{{H}_{3}})}_{5}}N{{O}_{2}}]C{{l}_{2}}\] is
AIEEE Solved Paper-2002
A)
optical
done
clear
B)
ionization
done
clear
C)
geometrical
done
clear
D)
linkage
done
clear
View Answer play_arrow
question_answer 52) One of the following equilibria is not affected by change in volume of the flask
AIEEE Solved Paper-2002
A)
\[PC{{l}_{5}}(g)\underset{{}}{\leftrightarrows}PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]
done
clear
B)
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\underset{\,}{\leftrightarrows}2N{{H}_{3}}(g)\]
done
clear
C)
\[{{N}_{2}}(g)+{{O}_{2}}(g)\overset{\,}{leftrightarrows}2NO(g)\]
done
clear
D)
\[S{{O}_{2}}C{{l}_{2}}(g)\underset{\,}{\leftrightarrows}S{{O}_{2}}(g)+C{{l}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 53) Uncertainty in position of a particle of 25 g in space is \[{{10}^{-5}}\] m. Hence, uncertainty in velocity \[(m{{s}^{-1}})\] is (Planck's constant \[h=6.6\times {{10}^{-34}}Js\])
AIEEE Solved Paper-2002
A)
\[2.1\times {{10}^{-28}}\]
done
clear
B)
\[2.1\times {{10}^{-34}}\]
done
clear
C)
\[0.5\times {{10}^{-34}}\]
done
clear
D)
\[5.0\times {{10}^{-24}}\]
done
clear
View Answer play_arrow
question_answer 54) Consider the following reactions at \[{{1100}^{o}}C\] (I) \[2C+{{O}_{2}}\xrightarrow{{}}2CO\], \[\Delta {{G}^{o}}=-460\,kJ\,mo{{l}^{-1}}\] (II) \[2Zn+{{O}_{2}}\xrightarrow{{}}2ZnO\], \[\Delta {{G}^{o}}+=-360\,kJ\,mo{{l}^{-1}}\] Based on these, select the correct alternate.
AIEEE Solved Paper-2002
A)
Zinc can be oxidised by CO
done
clear
B)
Zinc oxide can be reduced by carbon
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 55) A reaction is non-spontaneous at the freezing point of water but is spontaneous at the boiling point of water then
AIEEE Solved Paper-2002
A)
\[\Delta H\,\,\,\,\,+ve\] \[\Delta S\,\,\,\,+ve\]
done
clear
B)
\[\Delta H\,\,\,\,\,-ve\] \[\Delta S\,\,\,\,-ve\]
done
clear
C)
\[\Delta H\,\,\,\,\,-ve\] \[\Delta S\,\,\,\,+ve\]
done
clear
D)
\[\Delta H\,\,\,\,\,+ve\] \[\Delta S\,\,\,\,-ve\]
done
clear
View Answer play_arrow
question_answer 56) Monomers are converted to polymer by
AIEEE Solved Paper-2002
A)
hydrolysis of monomers
done
clear
B)
condensation reaction between monomers
done
clear
C)
protonation of monomers
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 57) Increasing order of bond strength of \[{{O}_{2}},O_{2}^{-}\,O_{2}^{2-}\] and \[O_{2}^{+}\] is
AIEEE Solved Paper-2002
A)
\[O_{2}^{+}<{{O}_{2}}<O_{2}^{-}<O_{2}^{2-}\]
done
clear
B)
\[{{O}_{2}}<O_{2}^{+}<O_{2}^{-}<O_{2}^{2-}\]
done
clear
C)
\[O_{2}^{-}<O_{2}^{2-}<O_{2}^{+}<{{O}_{2}}\]
done
clear
D)
\[O_{2}^{2-}<O_{2}^{-}<{{O}_{2}}<O_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 58) Most common oxidation states of Ce (cerium) are
AIEEE Solved Paper-2002
A)
\[+3,\,\,+4\]
done
clear
B)
\[+2,\,\,+3\]
done
clear
C)
\[+2,\,\,+4\]
done
clear
D)
\[+3,\,\,+5\]
done
clear
View Answer play_arrow
question_answer 59) \[C{{e}^{3+}},\,\,L{{a}^{3+}},\,P{{m}^{3+}}\] and \[Y{{b}^{3+}}\] have ionic radii in the increasing order as
AIEEE Solved Paper-2002
A)
\[L{{a}^{3+}}<C{{e}^{3+}}<P{{m}^{3+}}<Y{{b}^{3+}}\]
done
clear
B)
\[Y{{b}^{3+}}<P{{m}^{3+}}<C{{e}^{3+}}<L{{a}^{3+}}\]
done
clear
C)
\[L{{a}^{3+}}\,=C{{e}^{3+}}\,<P{{m}^{3+}}\,<Y{{b}^{3+}}\]
done
clear
D)
\[Y{{b}^{3+}}<P{{m}^{3+}}<L{{a}^{3+}}<C{{e}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 60) pH of \[0.005\] M calcium acetate (\[P{{K}_{a}}\] of \[C{{H}_{3}}COOH=4.74\]) is
AIEEE Solved Paper-2002
A)
\[7.04\]
done
clear
B)
\[9.37\]
done
clear
C)
\[9.26\]
done
clear
D)
\[8.37\]
done
clear
View Answer play_arrow
question_answer 61) \[{{H}_{2}}\] gas is absorbed on the metal surface like tungsten. This follows ...... order reaction.
AIEEE Solved Paper-2002
A)
third
done
clear
B)
second
done
clear
C)
zero
done
clear
D)
first
done
clear
View Answer play_arrow
question_answer 62) Rate constant k of the first order reaction when initial concentration \[{{C}_{0}}\] and concentration \[{{C}_{t}}\] at time \[t\] is given by equation \[kt=\log \,{{C}_{0}}-\log {{C}_{t}}\] Graph is a straight line if we plot
AIEEE Solved Paper-2002
A)
\[t\,\,vs\,\,\log \,\,{{C}_{0}}\]
done
clear
B)
\[t\,\,vs\,\,\log \,{{C}_{t}}\]
done
clear
C)
\[{{t}^{-1}}\,\,vs\,\,\log \,{{C}_{t}}\]
done
clear
D)
\[\log \,\,{{C}_{t}}\,vs\,\,\log \,\,{{C}_{t}}\]
done
clear
View Answer play_arrow
question_answer 63) Alum is widely used to purify water since
AIEEE Solved Paper-2002
A)
it forms complex with clay particles
done
clear
B)
it coagulates the mud particles
done
clear
C)
it exchanges \[C{{a}^{2+}}\] and \[M{{g}^{2+}}\] ions present in hard water
done
clear
D)
its sulphate ion is water purifier
done
clear
View Answer play_arrow
question_answer 64) On vigorous oxidation by permangnate solution \[{{(C{{H}_{3}})}_{2}}C=CHC{{H}_{2}}CHO\] gives
AIEEE Solved Paper-2002
A)
\[{{(C{{H}_{3}})}_{2}}CO\] and \[OHCC{{H}_{2}}CHO\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,HC{{H}_{2}}CHO\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CO\] and \[OHCC{{H}_{2}}COOH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CO\] and \[C{{H}_{2}}{{(COOH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 65)
In the following benzyl/allyl system \[R-CH=C{{H}_{2}}\] or
A)
\[{{(C{{H}_{3}})}_{3}}C->{{(C{{H}_{3}})}_{2}}CH->C{{H}_{3}}C{{H}_{2}}-\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}->{{(C{{H}_{3}})}_{2}}CH->{{(C{{H}_{3}})}_{3}}C-\]
done
clear
C)
\[(C{{H}_{3}}){{ }_{2}}CH->C{{H}_{3}}C{{H}_{2}}->{{(C{{H}_{3}})}_{3}}C-\]
done
clear
D)
\[(C{{H}_{3}}){{ }_{2}}C->C{{H}_{3}}C{{H}_{2}}->{{(C{{H}_{3}})}_{3}}CH-\]
done
clear
View Answer play_arrow
question_answer 66) \[PC{{l}_{3}}\] and \[PC{{l}_{5}}\], both exist; \[NC{{l}_{3}}\] exists but \[NC{{l}_{5}}\] does not exist. It is due to
AIEEE Solved Paper-2002
A)
lower electronegativity of P than N
done
clear
B)
lower tendency of N to form covalent bond
done
clear
C)
availability of vacant d-obital in P but not in N
done
clear
D)
statement is itself incorrect
done
clear
View Answer play_arrow
question_answer 67) Following types of compounds (as I, II) (I) \[C{{H}_{3}}CH=CHC{{H}_{3}}\] (II) \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{2}}C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-OH\] are studied in terms of isomerism in
AIEEE Solved Paper-2002
A)
chain isomerism
done
clear
B)
position isomerism
done
clear
C)
conformers
done
clear
D)
stereoisomerism
done
clear
View Answer play_arrow
question_answer 68) Conductivity (Seimen's S) is directly proportional to area of the vessel and the concentration of the solution in it and is inversely proportional to the length of the vessel, then constant of proportionality is expressed in
AIEEE Solved Paper-2002
A)
S m \[mo{{l}^{-1}}\]
done
clear
B)
\[{{S}^{2}}\,{{m}^{2}}\,mo{{l}^{-2}}\]
done
clear
C)
S \[{{m}^{2}}\,mo{{l}^{-1}}\]
done
clear
D)
\[{{S}^{2}}\,{{m}^{2}}\,mol\]
done
clear
View Answer play_arrow
question_answer 69) A heat engine absorbs heat \[{{q}_{1}}\] from a source at temperature \[{{T}_{1}}\] and heat \[{{q}_{2}}\] from a source at temperature \[{{T}_{2}}\]. Work done is found to be \[J({{q}_{1}}+{{q}_{2}})\] This is in accordance with
A)
first law of thermodynamics
done
clear
B)
second law of thermodynamics
done
clear
C)
Joules equivalent law
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 70) Select the correct statement.
AIEEE Solved Paper-2002
A)
When a covalent bond is formed, transfer of electrons takes place
done
clear
B)
Pure \[{{H}_{2}}O\] does not contain any ion
done
clear
C)
A bond is formed when attractive forces overcome repulsive forces
done
clear
D)
HF is less polar than \[HBr\]
done
clear
View Answer play_arrow
question_answer 71) The metallic sodium dissolves in liquid ammonia to form a deep blue coloured solution. The deep blue colour is due to formation of
AIEEE Solved Paper-2002
A)
solvated electron, \[{{e}^{-}}(N{{H}_{3}})_{x}^{-}\]
done
clear
B)
solvated atomic sodium, \[Na{{(N{{H}_{3}})}_{y}}\]
done
clear
C)
\[(N{{a}^{+}}+N{{a}^{-}})\]
done
clear
D)
\[NaN{{H}_{2}}+{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 72) Maximum dehydration takes place that of
AIEEE Solved Paper-2002
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
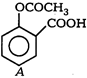
question_answer 73) \[{{S}_{N}}1\] reaction is feasible in
AIEEE Solved Paper-2002
A)
\[->-\,Cl+KOH\xrightarrow{{}}\]
done
clear
B)
\[+KOH\xrightarrow{{}}\]
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 74) Oxidation number of \[Cl\] in \[CaOC{{l}_{2}}\] (bleaching powder) is
AIEEE Solved Paper-2002
A)
zero, since it contains \[C{{l}_{2}}\]
done
clear
B)
-1, since it contains \[C{{l}^{-}}\]
done
clear
C)
+1, since it contains \[Cl{{O}^{-}}\]
done
clear
D)
+1 and -1 since it contains \[Cl{{O}^{-}}\] and \[C{{l}^{-}}\]
done
clear
View Answer play_arrow
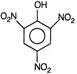
question_answer 75) Picric acid is
AIEEE Solved Paper-2002
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
 AIEEE Solved Paper-2002
AIEEE Solved Paper-2002