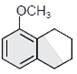
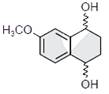
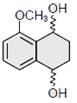
-
question_answer1) The correct statement about the synthesis of erythritol \[(C{{(C{{H}_{2}}OH)}_{4}})\]used in the preparation of PETN is:
JEE Main Online Paper (Held On 10 April 2016)
A)
The synthesis requires two aldol condensations and two Cannizzaro reactions.
done
clear
B)
Alpha hydrogens of ethanol and methanol are involved in this reaction.
done
clear
C)
The synthesis requires four aldol condensations between methanol and ethanol.
done
clear
D)
The synthesis requires three aldol condensations and one Cannizzaro reaction.
done
clear
View Answer play_arrow
-
question_answer2) The following statements concern elements in the periodic table. Which of the following is true?
JEE Main Online Paper (Held On 10 April 2016)
A)
The Group 13 elements are all metals.
done
clear
B)
All the elements in Group 17 are gases.
done
clear
C)
Elements of Group 16 have lower ionization enthalpy values compared to those of Group 15 in the corresponding periods.
done
clear
D)
For Group 15 elements, the stability of +5 oxidation state increases down the group.
done
clear
View Answer play_arrow
-
question_answer3) Identify the incorrect statement:
JEE Main Online Paper (Held On 10 April 2016)
A)
Rhombic and monoclinic sulphur have S8 molecules.
done
clear
B)
\[{{S}_{8}}\] ring has a crown shape.
done
clear
C)
\[{{S}_{2}}\] is paramagnetic like oxygen.
done
clear
D)
The S-S-S bond angles in the \[{{S}_{8}}\]and \[{{S}_{6}}\] rings are the same.
done
clear
View Answer play_arrow
-
question_answer4) Assertion: Among the carbon allotropes, diamond is an insulator, Whereas, graphite is a good conductor of electricity. Reason: Hybridization of carbon in diamond and graphite are \[s{{p}^{3}}\]and \[s{{p}^{2}},\]respectively.
JEE Main Online Paper (Held On 10 April 2016)
A)
Both assertion and reason are correct, but the reason is not the correct explanation for the assertion.
done
clear
B)
Assertion is incorrect statement, but the reason is correct.
done
clear
C)
Both assertion and reason are correct, and the reason is the correct explanation for the assertion.
done
clear
D)
Both assertion and reason are incorrect.
done
clear
View Answer play_arrow
-
question_answer5)
The "N" which does not contribute to the basicity for the compound is : 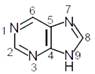 JEE Main Online Paper (Held On 10 April 2016)
JEE Main Online Paper (Held On 10 April 2016)
A)
N 7
done
clear
B)
N 1
done
clear
C)
N 9
done
clear
D)
N 3
done
clear
View Answer play_arrow
-
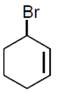
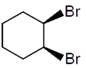
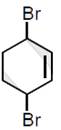
question_answer6)
Bromination of cyclohexene under conditions given below yields: 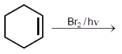 JEE Main Online Paper (Held On 10 April 2016)
JEE Main Online Paper (Held On 10 April 2016)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer7) The commercial name for calcium oxide is:
JEE Main Online Paper (Held On 10 April 2016)
A)
Quick lime
done
clear
B)
Milk of lime
done
clear
C)
Slaked lime
done
clear
D)
Limestone
done
clear
View Answer play_arrow
-
question_answer8)
Which one of the following reagents is not suitable for the elimination reaction ? 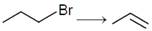 JEE Main Online Paper (Held On 10 April 2016)
JEE Main Online Paper (Held On 10 April 2016)
A)
NaI
done
clear
B)
\[NaOH/{{H}_{2}}O-EtOH\]
done
clear
C)
\[NaOH/{{H}_{2}}O\]
done
clear
D)
\[NaOEt/EtOH\]
done
clear
View Answer play_arrow
-
question_answer9) Which of the following is a bactericidal antibiotic?
A)
Erythromycin
done
clear
B)
Tetracycline
done
clear
C)
Ofloxacin
done
clear
D)
Chloramphenicol
done
clear
View Answer play_arrow
-
question_answer10) A solid XY kept in an evacuated sealed container undergoes decomposition to form a mixture of gases X and Y at temperature T. The equilibrium pressure is 10 bar in this vessel. \[{{K}_{P}}\] for this reaction is :
JEE Main Online Paper (Held On 10 April 2016)
A)
25
done
clear
B)
5
done
clear
C)
0
done
clear
D)
100
done
clear
View Answer play_arrow
-
question_answer11) The transition metal ions responsible for color in ruby and emerald are, respectively :
JEE Main Online Paper (Held On 10 April 2016)
A)
\[\text{C}{{\text{r}}^{\text{3+}}}\,\text{and}\,\,\text{C}{{\text{r}}^{\text{3+}}}\]
done
clear
B)
\[\text{C}{{\text{o}}^{\text{3+}}}\,\text{and}\,\,\text{C}{{\text{o}}^{\text{3+}}}\]
done
clear
C)
\[\text{C}{{\text{o}}^{\text{3+}}}\,\text{and}\,\,\text{C}{{\text{r}}^{\text{3+}}}\]
done
clear
D)
\[\text{C}{{\text{r}}^{\text{3+}}}\,\text{and}\,\,\text{C}{{\text{o}}^{\text{3+}}}\]
done
clear
View Answer play_arrow
-
question_answer12) Which of the following polymers is synthesized using a free radical polymerization technique?
JEE Main Online Paper (Held On 10 April 2016)
A)
Teflon
done
clear
B)
Melamine polymer
done
clear
C)
Nylon 6,6
done
clear
D)
Terylene
done
clear
View Answer play_arrow
-
question_answer13) Extraction of copper by smelting uses silica as an additive to remove :
JEE Main Online Paper (Held On 10 April 2016)
A)
FeS
done
clear
B)
FeO
done
clear
C)
\[C{{u}_{2}}S\]
done
clear
D)
\[C{{u}_{2}}O\]
done
clear
View Answer play_arrow
-
question_answer14) Initially, the root mean square (rms) velocity of \[{{N}_{2}}\]molecules at certain temperature is u. If this temperature is doubled and all the nitrogen molecules dissociate into nitrogen atoms, then the new rms velocity will be :
JEE Main Online Paper (Held On 10 April 2016)
A)
2 u
done
clear
B)
14 u
done
clear
C)
u / 2
done
clear
D)
4 u
done
clear
View Answer play_arrow
-
question_answer15) Gold numbers of some colloids are : Gelatin : 0.005 - 0.01, Gum Arabic : 0.15 - 0.25 ; Ole ate : 0.04 - 1.0 ; Starch : 15 - 25. Which among these is a better protective colloid ?
JEE Main Online Paper (Held On 10 April 2016)
A)
Gelatin
done
clear
B)
Starch
done
clear
C)
Gum Arabic
done
clear
D)
Ole ate
done
clear
View Answer play_arrow
-
question_answer16) Fluorination of an aromatic ring is easily accomplished by treating a diazonium salt with HBF4. Which of the following conditions is correct about this reaction?
JEE Main Online Paper (Held On 10 April 2016)
A)
Only heat
done
clear
B)
\[NaN{{O}_{2}}/Cu\]
done
clear
C)
\[C{{u}_{2}}O/{{H}_{2}}O\]
done
clear
D)
\[NaF/Cu\]
done
clear
View Answer play_arrow
-
question_answer17) Identify the correct statement:
JEE Main Online Paper (Held On 10 April 2016)
A)
Corrosion of iron can be minimized by forming an impermeable barrier at its surface.
done
clear
B)
Iron corrodes in oxygen-free water.
done
clear
C)
Iron corrodes more rapidly in salt water because its electrochemical potential is higher.
done
clear
D)
Corrosion of iron can be minimized by forming a contact with another metal with a higher reduction potential.
done
clear
View Answer play_arrow
-
question_answer18) An aqueous solution of a salt \[M{{X}_{2}}\] at certain temperature has a van't Hoff factor of 2. The degree of dissociation for this solution of the salt is :
JEE Main Online Paper (Held On 10 April 2016)
A)
0.67
done
clear
B)
0.33
done
clear
C)
0.80
done
clear
D)
0.50
done
clear
View Answer play_arrow
-
question_answer19) Oxidation of succinate ion produces ethylene and carbon dioxide gases. On passing 0.2 Faraday electricity through an aqueous solution of potassium succinate, the total volume of gases (at both cathode and anode) at STP (1 atm and 273 K) is :
JEE Main Online Paper (Held On 10 April 2016)
A)
6.72 L
done
clear
B)
2.24 L
done
clear
C)
4.48 L
done
clear
D)
8.96 L
done
clear
View Answer play_arrow
-
question_answer20) If 100 mole of \[{{H}_{2}}{{O}_{2}}\]decompose at 1 bar and 300 K, the work done (kJ) by one mole of\[{{O}_{2}}(g)\] as it expands against 1 bar pressure is : \[2{{H}_{2}}{{O}_{2}}(l)2{{H}_{2}}O(l)+{{O}_{2}}(g)\] \[(R=8.3J\,{{K}^{-1}}mo{{l}^{-1}})\]
JEE Main Online Paper (Held On 10 April 2016)
A)
498.00
done
clear
B)
62.25
done
clear
C)
124.50
done
clear
D)
249.00
done
clear
View Answer play_arrow
-
question_answer21) Which of the following is an example of homoleptic complex ?
JEE Main Online Paper (Held On 10 April 2016)
A)
\[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\]
done
clear
D)
\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]
done
clear
View Answer play_arrow
-
question_answer22) The bond angle H - X - H is the greatest in the compound :
JEE Main Online Paper (Held On 10 April 2016)
A)
\[N{{H}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[C{{H}_{4}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
-
question_answer23) The rate law for the reaction below is given by the expression k [A][B] \[A+B\to \]Product If the concentration of B is increased from 0.1 to 0.3 mole, keeping the value of A at 0.1 mole, the rate constant will be :
JEE Main Online Paper (Held On 10 April 2016)
A)
9 k
done
clear
B)
3 k
done
clear
C)
k/3
done
clear
D)
k
done
clear
View Answer play_arrow
-
question_answer24) Sodium extract is heated with concentrated \[HN{{O}_{3}}\] before testing for halogens because :
JEE Main Online Paper (Held On 10 April 2016)
A)
Ag reacts faster with halides in acidic medium.
done
clear
B)
Silver halides are totally insoluble in nitric acid.
done
clear
C)
\[A{{g}_{2}}S\]and \[AgCN\] are soluble in acidic medium.
done
clear
D)
\[{{S}^{2-}}\]and \[C{{N}^{-}},\]if present, are decomposed by conc. \[HN{{O}_{3}}\]and hence do not interfere in the test.
done
clear
View Answer play_arrow
-
question_answer25) Which one of the following substances used in dry cleaning is a better strategy to control environmental pollution?
JEE Main Online Paper (Held On 10 April 2016)
A)
Nitrogen dioxide
done
clear
B)
Sulphur dioxide
done
clear
C)
Tetrachloroethylene
done
clear
D)
Carbon dioxide
done
clear
View Answer play_arrow
-
question_answer26)
Consider the reaction sequence below : 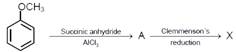 JEE Main Online Paper (Held On 10 April 2016)
JEE Main Online Paper (Held On 10 April 2016)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer27) Observation of "Rhumann's purple" is a confirmatory test for the presence of :
JEE Main Online Paper (Held On 10 April 2016)
A)
Reducing sugar
done
clear
B)
Starch
done
clear
C)
Protein
done
clear
D)
Cupric ion
done
clear
View Answer play_arrow
-
question_answer28) Aqueous solution of which salt will not contain ions with the electronic configuration \[1{{s}^{2}}2{{s}^{2}}{{p}^{6}}3{{s}^{2}}3{{p}^{6}}\] ?
JEE Main Online Paper (Held On 10 April 2016)
A)
\[NaCl\]
done
clear
B)
\[Ca{{I}_{2}}\]
done
clear
C)
\[NaF\]
done
clear
D)
\[KBr\]
done
clear
View Answer play_arrow
-
question_answer29) The volume of 0.1 N dibasic acid sufficient to neutralize 1 g of a base that furnishes 0.04 mole of \[O{{H}^{-}}\] in aqueous solution is :
JEE Main Online Paper (Held On 10 April 2016)
A)
400 mL
done
clear
B)
600 mL
done
clear
C)
200 mL
done
clear
D)
80 mL
done
clear
View Answer play_arrow
-
question_answer30) Identify the reaction which does not liberate hydrogen :
JEE Main Online Paper (Held On 10 April 2016)
A)
Allowing a solution of sodium in liquid ammonia to stand.
done
clear
B)
Reaction of zinc with aqueous alkali.
done
clear
C)
Reaction of lithium hydride with \[{{B}_{2}}{{H}_{6}}.\]
done
clear
D)
Electrolysis of acidified water using Pt electrodes.
done
clear
View Answer play_arrow
 JEE Main Online Paper (Held On 10 April 2016)
JEE Main Online Paper (Held On 10 April 2016)
 JEE Main Online Paper (Held On 10 April 2016)
JEE Main Online Paper (Held On 10 April 2016)
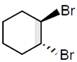
 JEE Main Online Paper (Held On 10 April 2016)
JEE Main Online Paper (Held On 10 April 2016)