-
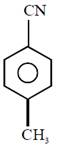
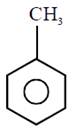
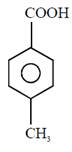
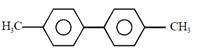
question_answer1) Which compound would give 5-keto-2-methyl hexanal upon ozonolysis ?
[JEE Main Solved Paper-2015 ]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer2) Which of the vitamins given below is water soluble ?
[JEE Main Solved Paper-2015 ]
A)
Vitamin E
done
clear
B)
Vitamin K
done
clear
C)
Vitamin C
done
clear
D)
Vitamin D
done
clear
View Answer play_arrow
-
question_answer3) Which one of the following alkaline earth metal sulphates has its hydration enthalpy greater than its lattice enthalpy ?
[JEE Main Solved Paper-2015 ]
A)
\[BaS{{O}_{4}}\]
done
clear
B)
\[SrS{{O}_{4}}\]
done
clear
C)
\[CaS{{O}_{4}}\]
done
clear
D)
\[BeS{{O}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer4)
In the reaction 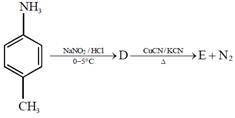 the product E is :
[JEE Main Solved Paper-2015 ]
the product E is :
[JEE Main Solved Paper-2015 ]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer5) Sodium metal crystallizes in a body centred cubic lattice with a unit cell edge of \[4.29\overset{\text{o}}{\mathop{\text{A}}}\,\]. The radius of sodium atom is approximately :
[JEE Main Solved Paper-2015 ]
A)
\[5.72\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[0.93\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1.86\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[3.22\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
-
question_answer6) Which of the following compounds is not colored yellow ?
[JEE Main Solved Paper-2015 ]
A)
\[{{(N{{H}_{4}})}_{3}}[As{{(M{{o}_{3}}{{O}_{10}})}_{4}}]\]
done
clear
B)
\[BaCr{{O}_{4}}\]
done
clear
C)
\[Z{{n}_{2}}[Fe{{(CN)}_{6}}]\]
done
clear
D)
\[{{K}_{3}}[Co{{(N{{O}_{2}})}_{6}}\]
done
clear
View Answer play_arrow
-
question_answer7) Which of the following is the energy of a possible excited state of hydrogen ?
[JEE Main Solved Paper-2015 ]
A)
- 3.4 eV
done
clear
B)
+ 6.8 eV
done
clear
C)
+ 13.6 eV
done
clear
D)
- 6.8 eV
done
clear
View Answer play_arrow
-
question_answer8) Which of the following compounds is not an antacid ?
[JEE Main Solved Paper-2015 ]
A)
Phenelzine
done
clear
B)
Ranitidine
done
clear
C)
Aluminium hydroxide
done
clear
D)
Cimetidine
done
clear
View Answer play_arrow
-
question_answer9) The ionic radii (in Å) of \[{{N}^{3-}},{{O}^{2-}}\]and \[{{F}^{-}}\] are respectively :
[JEE Main Solved Paper-2015 ]
A)
1.71, 1.40 and 1.36
done
clear
B)
1.71, 1.36 and 1.40
done
clear
C)
1.36, 1.40 and 1.71
done
clear
D)
1.36, 1.71 and 1.40
done
clear
View Answer play_arrow
-
question_answer10) In the context of the Hall-Heroult process for the extraction of Al, which of the following statements is false?
[JEE Main Solved Paper-2015 ]
A)
\[A{{l}^{3+}}\] is reduced at the cathode to form Al
done
clear
B)
\[N{{a}_{3}}Al{{F}_{6}}\] serves as the electrolyte
done
clear
C)
\[CO\] and \[C{{O}_{2}}\] are produced in this process
done
clear
D)
\[A{{l}_{2}}{{O}_{3}}\]is mixed with \[Ca{{F}_{2}}\] which lowers the melting point of the mixture and brings conductivity
done
clear
View Answer play_arrow
-
question_answer11) In the following sequence of reactions : Toluene \[\xrightarrow[{}]{KMn{{O}_{4}}}A\xrightarrow[{}]{SOC{{l}_{2}}}B\xrightarrow[BaS{{O}_{4}}]{{{H}_{2}}/Pd}C,\]the product C is:
[JEE Main Solved Paper-2015 ]
A)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}COOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer12) Higher order (>3) reactions are rare due to :
[JEE Main Solved Paper-2015 ]
A)
shifting of equilibrium towards reactants due to elastic collisions
done
clear
B)
loss of active species on collision
done
clear
C)
low probability of simultaneous collision of all the reacting species
done
clear
D)
increase in entropy and activation energy as more molecules are involved
done
clear
View Answer play_arrow
-
question_answer13) Which of the following compounds will exhibit geometrical isomerism?
[JEE Main Solved Paper-2015 ]
A)
2-Phenyl-1-butene
done
clear
B)
1,1-Diphenyl-1-propane
done
clear
C)
1-Phenyl-2-butene
done
clear
D)
3-Phenyl-1-butene
done
clear
View Answer play_arrow
-
question_answer14)
Match the catalysts to the correct processes:
[JEE Main Solved Paper-2015 ]
| Catalyst |
Process |
| (a) \[TiC{{l}_{3}}\] |
(i) Wacker process |
| (b) \[PdC{{l}_{2}}\] |
(ii) Ziegler - Natta polymerization |
| (c) \[CuC{{l}_{2}}\] |
(iii) Contact process |
| (d) \[{{V}_{2}}{{O}_{5}}\] |
(iv) Deacon?s process |
A)
(A)-(ii), (B)-(iii), (C)-(iv), (D) -(i)
done
clear
B)
(A)-(iii), (B)-(i), (C)-(ii), (D) -(iv)
done
clear
C)
(A)-(iii), (B)-(ii), (C)-(iv), (D) -(i)
done
clear
D)
(A)-(ii), (B)-(i), (C)-(iv), (D) -(iii)
done
clear
View Answer play_arrow
-
question_answer15) The intermolecular interaction that is dependent on the inverse cube of distance between the molecules is:
[JEE Main Solved Paper-2015 ]
A)
London force
done
clear
B)
hydrogen bond
done
clear
C)
ion- ion interaction
done
clear
D)
ion-dipole interaction
done
clear
View Answer play_arrow
-
question_answer16) The molecular formula of a commercial resin used for exchanging ions in water softening is \[{{C}_{8}}{{H}_{7}}S{{O}_{3}}Na\] (Mol. wt. 206). \[C{{a}^{2+}}\] ions by the resin when expressed in mole per gram resin ?
[JEE Main Solved Paper-2015 ]
A)
\[\frac{2}{309}\]
done
clear
B)
\[\frac{1}{412}\]
done
clear
C)
\[\frac{1}{103}\]
done
clear
D)
\[\frac{1}{206}\]
done
clear
View Answer play_arrow
-
question_answer17) Two Faraday of electricity is passed through a solution of \[CuS{{O}_{4}}.\] The mass of copper deposited at the cathode is : (at. mass of Cu = 63.5 amu)
[JEE Main Solved Paper-2015 ]
A)
2 g
done
clear
B)
127 g
done
clear
C)
0 g
done
clear
D)
63.5 g
done
clear
View Answer play_arrow
-
question_answer18) The number of geometric isomers that can exist for square planar \[{{\left[ Pt\left( Cl \right)\left( py \right)\left( N{{H}_{3}} \right)\left( N{{H}_{2}}OH \right) \right]}^{+}}\] is \[\text{(py}\,\text{=}\,\,\text{pyridine):}\]
[JEE Main Solved Paper-2015 ]
A)
4
done
clear
B)
6
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
-
question_answer19) Carius method of estimation of halogens, 250 mg of an organic compound gave 141 mg of AgBr. The percentage of bromine in the compound is: (at. mass Ag = 108; Br = 80)
[JEE Main Solved Paper-2015 ]
A)
48
done
clear
B)
60
done
clear
C)
24
done
clear
D)
36
done
clear
View Answer play_arrow
-
question_answer20) The colour of \[KMn{{O}_{4}}\] is due to:
[JEE Main Solved Paper-2015 ]
A)
\[L\to M\] charge transfer transition
done
clear
B)
\[\sigma \to \sigma *\] transition
done
clear
C)
\[M\to L\] charge transfer transition
done
clear
D)
\[d-d\] transition
done
clear
View Answer play_arrow
-
question_answer21) The synthesis of alkyl fluorides is best accomplished by :
[JEE Main Solved Paper-2015 ]
A)
Finkelstein reaction
done
clear
B)
Swarts reaction
done
clear
C)
Free radical fluorination
done
clear
D)
Sandmeyer's reaction
done
clear
View Answer play_arrow
-
question_answer22) 3g of activated charcoal was added to 50mL acetic solution (0.06N) in a flask. After an hour it was filtered and the strength of the filtrate was found to be 0.042 N. The amount of acetic acid adsorbed (per gram charcoal) is :
[JEE Main Solved Paper-2015 ]
A)
42 mg
done
clear
B)
54 mg
done
clear
C)
18 mg
done
clear
D)
36 mg
done
clear
View Answer play_arrow
-
question_answer23) The vapour pressure of acetone at 200C is 185 torr. When 1.2 g of a non ? volatile substance was dissolved in 100 g of acetone at 200C, its vapour pressure was 183 torr. The molar mass (g mol?1) of the substance is
[JEE Main Solved Paper-2015 ]
A)
128
done
clear
B)
488
done
clear
C)
32
done
clear
D)
64
done
clear
View Answer play_arrow
-
question_answer24) Which among the following is the most reactive ?
[JEE Main Solved Paper-2015 ]
A)
\[{{I}_{2}}\]
done
clear
B)
\[ICl\]
done
clear
C)
\[C{{l}_{2}}\]
done
clear
D)
\[B{{r}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer25) The standard Gibbs energy change at 300K for the reaction \[2AB+C\]At a given time, the composition of the reaction mixture is\[\left[ A \right]=\frac{1}{2},\left[ B \right]=2\]and \[\left[ C \right]=\frac{1}{2}.\] The reaction proceeds in the : \[[R=8.314J/Kmol,e=2.718]\]
[JEE Main Solved Paper-2015 ]
A)
forward direction because \[Q<{{K}_{C}}\]
done
clear
B)
reverse direction because \[Q<{{K}_{C}}\]
done
clear
C)
forward direction because \[Q>{{K}_{C}}\]
done
clear
D)
reverse direction beacuse \[Q>{{K}_{C}}\]
done
clear
View Answer play_arrow
-
question_answer26) Assertion : Nitrogen and oxygen are the main components in the atmosphere but these do not react to form oxides of nitrogen. Reason : The reaction between nitrogen and oxygen requires high temperature.
[JEE Main Solved Paper-2015 ]
A)
The assertion is incorrect, but the reason is correct
done
clear
B)
Both the assertion and reason are incorrect
done
clear
C)
Both assertion and reason are correct, and the reason is the correct explanation for the assertion
done
clear
D)
Both assertion and reason are correct, but reason is not the correct explanation for the assertion
done
clear
View Answer play_arrow
-
question_answer27) Which one has the highest boiling point ?
[JEE Main Solved Paper-2015 ]
A)
Kr
done
clear
B)
Xe
done
clear
C)
He
done
clear
D)
Ne
done
clear
View Answer play_arrow
-
question_answer28) Which polymer is used in the manufacture of paints and lacquers ?
[JEE Main Solved Paper-2015 ]
A)
Polypropene
done
clear
B)
Poly vinyl chloride
done
clear
C)
Bakelite
done
clear
D)
Glyptal
done
clear
View Answer play_arrow
-
question_answer29) The following reaction is performed at \[298K.2NO(g)+{{O}_{2}}2N{{O}_{2}}(g)\] The standard free energy of formation of \[NO(g)\] is 86.6 kJ/mol at 298 K. What is the standard free energy of formation of \[N{{O}_{2}}(g)\] at 298K? \[\left( {{K}_{p}}=1.6\times {{10}^{12}} \right)\]
[JEE Main Solved Paper-2015 ]
A)
\[86600-\frac{\ln \left( 1.6\times {{10}^{12}} \right)}{R\left( 298 \right)}\]
done
clear
B)
\[0.5\left[ 2\times 86,600-R\left( 298 \right)\ln \left( 1.6\times {{10}^{12}} \right) \right]\]
done
clear
C)
\[R\left( 298 \right)\ln \left( 1.6\times {{10}^{12}} \right)-86600\]
done
clear
D)
\[86600+R\left( 298 \right)\ln \left( 1.6\times {{10}^{12}} \right)\]
done
clear
View Answer play_arrow
-
question_answer30) From the following statements regarding \[{{H}_{2}}{{O}_{2}},\] choose the incorrect statement :
[JEE Main Solved Paper-2015 ]
A)
It has to be stored in plastic or wax lined glass bottles in dark
done
clear
B)
It has to be kept away from dust
done
clear
C)
It can act only as an oxidizing agent
done
clear
D)
It decomposes on exposure to light
done
clear
View Answer play_arrow
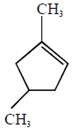
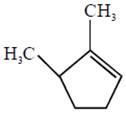
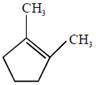
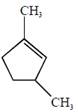
 the product E is :
[JEE Main Solved Paper-2015 ]
the product E is :
[JEE Main Solved Paper-2015 ]