-
question_answer1) Ionization energy of gaseous Na atoms is \[495.5\,\text{kJ}\,\text{mo}{{\text{l}}^{-1}}\] The lowest possible frequency of light that ionizes a sodium atom is \[(h=6.626\times {{10}^{-34}}Js,{{N}_{A}}=6.022\times {{10}^{23}}mo{{l}^{-1}})\]
JEE Main Online Paper (Held On 19 April 2016)
A)
\[7.50\times {{10}^{4}}{{s}^{-1}}\]
done
clear
B)
\[4.76\times {{10}^{14}}{{s}^{-1}}\]
done
clear
C)
\[3.15\times {{10}^{15}}{{s}^{-1}}\]
done
clear
D)
\[1.24\times {{10}^{15}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer2) Choose the correct statement with respect to the vapour pressure of a liquid among the following:
JEE Main Online Paper (Held On 19 April 2016)
A)
Increases linearly with increasing temperature
done
clear
B)
Increases non-linearly with increasing temperature
done
clear
C)
Decreases linearly with increasing temperature
done
clear
D)
Decreases non-linearly with increasing temperature
done
clear
View Answer play_arrow
-
question_answer3) Which one of the following molecules is paramagnetic?
JEE Main Online Paper (Held On 19 April 2016)
A)
\[{{N}_{2}}\]
done
clear
B)
NO
done
clear
C)
CO
done
clear
D)
\[{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer4) Zirconium phosphate \[[Z{{r}_{3}}{{(P{{O}_{4}})}_{4}}]\]dissociates into three zirconium cations of charge + 4 and four phosphate anions of charge ? 3. If molar solubility of zirconium phosphate is denoted by S and its solubility product by \[{{K}_{sp}}\] then which of the following relationship between S and \[{{K}_{sp}}\]is correct?
JEE Main Online Paper (Held On 19 April 2016)
A)
\[S=\{{{K}_{sp}}/{{(6912)}^{1/7}}\}\]
done
clear
B)
\[S={{\{{{K}_{sp}}/144\}}^{1/7}}\]
done
clear
C)
\[S={{\{{{K}_{sp}}/6912\}}^{1/7}}\]
done
clear
D)
\[S={{\{{{K}_{sp}}/6912\}}^{7}}\]
done
clear
View Answer play_arrow
-
question_answer5) For the decomposition of the compound, represented as\[N{{H}_{2}}COON{{H}_{4}}(s)2N{{H}_{3}}(g)+C{{O}_{2}}(g)\] the \[{{K}_{p}}=2.9\times {{10}^{-5}}at{{m}^{3}}.\] If the reaction is started with 1 mol of the compound, the total pressure at equilibrium would be :
JEE Main Online Paper (Held On 19 April 2016)
A)
\[1.94\times {{10}^{-2}}atm\]
done
clear
B)
\[5.82\times {{10}^{-2}}atm\]
done
clear
C)
\[7.66\times {{10}^{-2}}atm\]
done
clear
D)
\[38.8\times {{10}^{-2}}atm\]
done
clear
View Answer play_arrow
-
question_answer6) For the reaction, \[3A+2B\to C+D,\] the differential rate law can be written as:
JEE Main Online Paper (Held On 19 April 2016)
A)
\[\frac{1}{3}\frac{d\left[ A \right]}{dt}=\frac{d\left[ C \right]}{dt}=k{{\left[ A \right]}^{n}}{{\left[ B \right]}^{m}}\]
done
clear
B)
\[-\frac{d\left[ A \right]}{dt}=\frac{d\left[ C \right]}{dt}=k{{\left[ A \right]}^{n}}{{\left[ B \right]}^{m}}\]
done
clear
C)
\[+\frac{1}{3}\frac{d\left[ A \right]}{dt}=-\frac{d\left[ C \right]}{dt}=k{{\left[ A \right]}^{n}}{{\left[ B \right]}^{m}}\]
done
clear
D)
\[-\frac{1}{3}\frac{d\left[ A \right]}{dt}=\frac{d\left[ C \right]}{dt}=k{{\left[ A \right]}^{n}}{{\left[ B \right]}^{m}}\]
done
clear
View Answer play_arrow
-
question_answer7) Sulphur dioxide and oxygen were allowed to diffuse through a porous partition. \[20d{{m}^{3}}\]of \[S{{O}_{2}}\] diffuses through the porous partition in 60 seconds. The volume of \[{{O}_{2}}\] in \[d{{m}^{3}}\]which diffuses under the similar condition in 30 seconds will be (atomic mass of sulphur = 32 u):
JEE Main Online Paper (Held On 19 April 2016)
A)
7.09
done
clear
B)
14.1
done
clear
C)
10.0
done
clear
D)
28.2
done
clear
View Answer play_arrow
-
question_answer8) The observed osmotic pressure for a 0.10 M solution of \[Fe{{(N{{H}_{4}})}_{2}}{{(S{{O}_{4}})}_{2}}\]at 25°C is 10.8 atm. The expected and experimental (observed) values of van?t Hoff factor (i) will be respectively: \[(R=0.082Lamt\,{{k}^{-1}}mo{{l}^{-1}})\]
JEE Main Online Paper (Held On 19 April 2016)
A)
5 and 4.42
done
clear
B)
4 and 4.00
done
clear
C)
5 and 3.42
done
clear
D)
3 and 5.42
done
clear
View Answer play_arrow
-
question_answer9) The total number of octahedral void (s) per atom present in a cubic close packed structure is:
JEE Main Online Paper (Held On 19 April 2016)
A)
2
done
clear
B)
4
done
clear
C)
1
done
clear
D)
3
done
clear
View Answer play_arrow
-
question_answer10) For an ideal solution of two components A and B, which of the following is true?
JEE Main Online Paper (Held On 19 April 2016)
A)
\[\Delta {{H}_{mixing}}<0\] (zero)
done
clear
B)
\[\Delta {{H}_{mixing}}>0\] (zero)
done
clear
C)
A - B interaction is stronger than A - A and B - B interactions
done
clear
D)
A - A, B - B and A - B interactions are identical.
done
clear
View Answer play_arrow
-
question_answer11) Consider the reaction: \[{{H}_{2}}S{{O}_{3}}(aq)+S{{n}^{4+}}(aq)+{{H}_{2}}O(l)\] \[\to S{{n}^{2+}}(aq)+HSO_{4}^{-}(aq)+3{{H}^{+}}(aq)\] Which of the following statements is correct?
JEE Main Online Paper (Held On 19 April 2016)
A)
\[S{{n}^{4+}}\]is the oxidizing agent because it undergoes oxidation
done
clear
B)
\[S{{n}^{4+}}\]is the reducing agent because it undergoes oxidation
done
clear
C)
\[{{H}_{2}}S{{O}_{3}}\]is the reducing agent because it undergoes oxidation
done
clear
D)
\[{{H}_{2}}S{{O}_{3}}\]is the reducing agent because it undergoes reduction
done
clear
View Answer play_arrow
-
question_answer12) Which one of the following has largest ionic radius?
JEE Main Online Paper (Held On 19 April 2016)
A)
\[L{{i}^{+}}\]
done
clear
B)
\[O_{2}^{2-}\]
done
clear
C)
\[{{B}^{3+}}\]
done
clear
D)
\[{{F}^{-}}\]
done
clear
View Answer play_arrow
-
question_answer13) An octahedral complex with molecular composition \[N{{H}_{3}}.Cl.S{{O}_{4}}\]has two isomers, A and B. The solution of A gives a white precipitate with \[AgN{{O}_{3}}\] solution and the solution of B gives white precipitate with \[BaC{{l}_{2}}\] solution. The type of isomerism exhibited by the complex is:
JEE Main Online Paper (Held On 19 April 2016)
A)
Linkage isomerism
done
clear
B)
Ionisation isomerism
done
clear
C)
Coordinate isomerism
done
clear
D)
Geometrical isomerism
done
clear
View Answer play_arrow
-
question_answer14) How many electrons are involved in the following redox reaction? \[C{{r}_{2}}O_{7}^{2-}+F{{e}^{2+}}+{{C}_{2}}O_{4}^{2-}\]\[\to C{{r}^{3+}}+F{{e}^{3+}}+C{{O}_{2}}\](Unbalanced)
JEE Main Online Paper (Held On 19 April 2016)
A)
3
done
clear
B)
4
done
clear
C)
6
done
clear
D)
5
done
clear
View Answer play_arrow
-
question_answer15) Amongst \[LiCl,RbCl,BeC{{l}_{2}}\] and \[MgC{{l}_{2}}\] the compounds with the greatest and the least ionic character, respectively are:
JEE Main Online Paper (Held On 19 April 2016)
A)
LiCl and RbCl
done
clear
B)
RbCl and \[BeC{{l}_{2}}\]
done
clear
C)
\[MgC{{l}_{2}}\] and \[BeC{{l}_{2}}\]
done
clear
D)
RbCl and \[MgC{{l}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer16) Nickel (Z = 28) combines with a uninegative monodentate ligand to form a diamagnetic complex \[{{[Ni{{L}_{4}}]}^{2-}}.\]The hybridisation involved and the number of unpaired electrons present in the complex are respectively:
JEE Main Online Paper (Held On 19 April 2016)
A)
\[s{{p}^{3}},\] two
done
clear
B)
\[ds{{p}^{2}},\]zero
done
clear
C)
\[ds{{p}^{2}},\]one
done
clear
D)
\[s{{p}^{3}},\]zero
done
clear
View Answer play_arrow
-
question_answer17) Which of these statements is not true?
JEE Main Online Paper (Held On 19 April 2016)
A)
\[N{{O}^{+}}\]is not isoelectronic with\[{{O}_{2}}\]
done
clear
B)
B is always covalent in its compounds
done
clear
C)
In aqueous solution, the \[T{{l}^{+}}\] ion is much more stable than Tl (III)
done
clear
D)
\[LiAl{{H}_{4}}\]is a versatile reducing agent in organic synthesis.
done
clear
View Answer play_arrow
-
question_answer18) Example of a three-dimensional silicate is:
JEE Main Online Paper (Held On 19 April 2016)
A)
Zeolites
done
clear
B)
Ultramarines
done
clear
C)
Feldspars
done
clear
D)
Beryls
done
clear
View Answer play_arrow
-
question_answer19) Amongst the following, identify the species with an atom in + 6 oxidation state:
JEE Main Online Paper (Held On 19 April 2016)
A)
\[{{[Mn{{O}_{4}}]}^{-}}\]
done
clear
B)
\[{{[Cr{{(CN)}_{6}}]}^{3-}}\]
done
clear
C)
\[C{{r}_{2}}{{O}_{3}}\]
done
clear
D)
\[Cr{{O}_{2}}C{{l}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer20) Which one of the following ores is known as Malachite:
JEE Main Online Paper (Held On 19 April 2016)
A)
\[C{{u}_{2}}O\]
done
clear
B)
\[C{{u}_{2}}S\]
done
clear
C)
\[CuFe{{S}_{2}}\]
done
clear
D)
\[Cu{{(OH)}_{2}}.CuC{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer21) The major product formed when 1, 1, 1-trichloro-propane is treated with aqueous potassium hydroxide is:
JEE Main Online Paper (Held On 19 April 2016)
A)
Propyne
done
clear
B)
1-Propanol
done
clear
C)
2-Propanol
done
clear
D)
Propionic acid
done
clear
View Answer play_arrow
-
question_answer22) Which one of the following is an example of thermosetting polymers?
JEE Main Online Paper (Held On 19 April 2016)
A)
Neoprene
done
clear
B)
Buna-N
done
clear
C)
Nylon 6, 6
done
clear
D)
Bakelite
done
clear
View Answer play_arrow
-
question_answer23)
The correct IUPAC name of the following compound is:  JEE Main Online Paper (Held On 19 April 2016)
JEE Main Online Paper (Held On 19 April 2016)
A)
4 - methyl - 3 - ethylhexane
done
clear
B)
3 - ethyl - 4 - methylhexane
done
clear
C)
3, 4 - ethylmethylhexane
done
clear
D)
4 - ethyl - 3 - methylhexane
done
clear
View Answer play_arrow
-
question_answer24)
Which one of the following substituents at para-position is most effective in stabilizing the phenoxide O - ion? 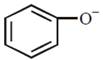 JEE Main Online Paper (Held On 19 April 2016)
JEE Main Online Paper (Held On 19 April 2016)
A)
\[-C{{H}_{3}}\]
done
clear
B)
\[-OC{{H}_{3}}\]
done
clear
C)
\[-COC{{H}_{3}}\]
done
clear
D)
\[-C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
-
question_answer25) The final product formed when Methyl amine is treated with \[NaN{{O}_{2}}\] and HCl is:
JEE Main Online Paper (Held On 19 April 2016)
A)
Diazomethane
done
clear
B)
Methylalcohol
done
clear
C)
Methylcyanide
done
clear
D)
Nitromethane
done
clear
View Answer play_arrow
-
question_answer26) Which one of the following compounds will not be soluble in sodium bicarbonate?
JEE Main Online Paper (Held On 19 April 2016)
A)
2, 4, 6 - Trinitrophenol
done
clear
B)
Benzoic acid
done
clear
C)
o-Nitrophenol
done
clear
D)
Benzene sulphonic acid
done
clear
View Answer play_arrow
-
question_answer27) Williamson synthesis of ether is an example of:
JEE Main Online Paper (Held On 19 April 2016)
A)
Nucleophilic addition
done
clear
B)
Electrophilic addition
done
clear
C)
Electrophilic substitution
done
clear
D)
Nucleophilic substitution
done
clear
View Answer play_arrow
-
question_answer28) The reason for double helical structure of DNA is the operation of:
JEE Main Online Paper (Held On 19 April 2016)
A)
Electrostatic attractions
done
clear
B)
van der Waals forces
done
clear
C)
Dipole - Dipole interactions
done
clear
D)
Hydrogen bonding
done
clear
View Answer play_arrow
-
question_answer29) Among the following organic acids, the acid present in rancid butter is:
JEE Main Online Paper (Held On 19 April 2016)
A)
Pyruvic acid
done
clear
B)
Lactic acid
done
clear
C)
Butyric acid
done
clear
D)
Acetic acid
done
clear
View Answer play_arrow
-
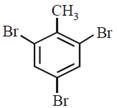
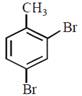
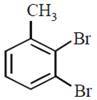
question_answer30)
In a set of reactions p-nitrotoluene yielded a product E. 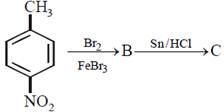 \[\xrightarrow[HCl]{NaN{{O}_{2}}}D\xrightarrow[HBr]{CuBr}E\] The product E would be:
JEE Main Online Paper (Held On 19 April 2016)
\[\xrightarrow[HCl]{NaN{{O}_{2}}}D\xrightarrow[HBr]{CuBr}E\] The product E would be:
JEE Main Online Paper (Held On 19 April 2016)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
 JEE Main Online Paper (Held On 19 April 2016)
JEE Main Online Paper (Held On 19 April 2016)
 JEE Main Online Paper (Held On 19 April 2016)
JEE Main Online Paper (Held On 19 April 2016)
 \[\xrightarrow[HCl]{NaN{{O}_{2}}}D\xrightarrow[HBr]{CuBr}E\] The product E would be:
JEE Main Online Paper (Held On 19 April 2016)
\[\xrightarrow[HCl]{NaN{{O}_{2}}}D\xrightarrow[HBr]{CuBr}E\] The product E would be:
JEE Main Online Paper (Held On 19 April 2016)