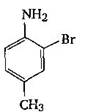
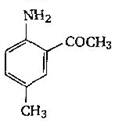
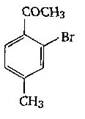
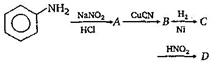
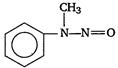
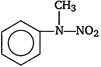
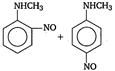
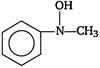
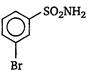
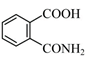
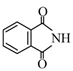
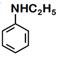
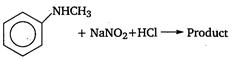question_answer 1) Aniline is reacted with bromine water and the resulting product is treated with an aqueous solution of sodium nitrite in presence of dilute hydrochloric acid. The compound so formed is converted to a tetrafluoroborate which is subsequently heated. The final product is: [AIPMT 1998]
A)
1, 3, 5-tribromobenzene
done
clear
B)
p-bromofluorobenzene
done
clear
C)
p-bromoaniline
done
clear
D)
2, 4, 6-tribromofluorobenzene
done
clear
View Answer play_arrow
question_answer 2) The number of molecules of ATP produced in the lipid metabolism of a molecule of palmitic acid is: [AIPMT 1998]
A)
130
done
clear
B)
36
done
clear
C)
56
done
clear
D)
86
done
clear
View Answer play_arrow
question_answer 3) In DNA the complementary bases are: [AIPMT 1998]
A)
adenine and thymine, guanine and cytosine
done
clear
B)
uracil and adenine, cytosine and guanine
done
clear
C)
adenine and guanine, thymine and cytosine
done
clear
D)
adenine and thymine, guanine and uracil
done
clear
View Answer play_arrow
question_answer 4) In the reaction \[C{{H}_{3}}CN+2H\xrightarrow[SnC{{l}_{2}}]{HCl}X\xrightarrow[{}]{\text{Boiling}\,{{H}_{2}}O}Y,\] the term Y is: [AIPMT 1999]
A)
acetone
done
clear
B)
ethanamine
done
clear
C)
acetaldehyde
done
clear
D)
dimethyl amine
done
clear
View Answer play_arrow
question_answer 5) Phenyl isocyanides are prepared from which of the following reaction? [AIPMT 1999]
A)
Rosenmund's reaction
done
clear
B)
Carbylamine reaction
done
clear
C)
Reimcr-Tiemann reaction
done
clear
D)
Wurtz reaction
done
clear
View Answer play_arrow
question_answer 6) Amides can be converted into amines by a reaction named after: [AIPMT 1999]
A)
Perkin
done
clear
B)
Claisen
done
clear
C)
Hofmann
done
clear
D)
Kekule
done
clear
View Answer play_arrow
question_answer 7) An organic compound A on reduction gives compound B which on reaction with chloroform and potassium hydroxide forms C. The compound C on catalytic reduction gives N-methylaniline. The compound A is: [AIPMT 2000]
A)
nitrobenzene
done
clear
B)
nitromethane
done
clear
C)
methylamine
done
clear
D)
aniline
done
clear
View Answer play_arrow
question_answer 8) Intermediates formed during reaction of \[\underset{\begin{smallmatrix} |\,\,| \\ O \end{smallmatrix}}{\mathop{RCN{{H}_{2}}}}\,\] with \[B{{r}_{2}}\] and \[KOH\] are: [AIPMT 2001]
A)
\[RCONHBr\] and \[RNCO\]
done
clear
B)
\[RNHCOBr\] and \[RNCO\]
done
clear
C)
\[RNHBra\] and\[KCONHBr\]
done
clear
D)
\[RCONB{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 9) \[\begin{align} & C{{H}_{3}}C{{H}_{2}}Cl\xrightarrow{NaCN}X\xrightarrow[{}]{Ni/{{H}_{2}}}Y- \\ & | \\ & Z\xleftarrow{\text{Acetic anhydride}}| \\ \end{align}\] [AIPMT 2002]
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}NHCOC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CONHC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CONHCOC{{H}_{3}}\]
done
clear
View Answer play_arrow
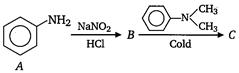
question_answer 10)
The final product C, obtained in this reaction [AIPMT 2003]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
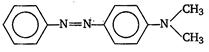
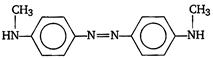
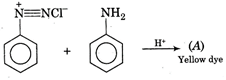
question_answer 11) Aniline when diazotised in cold and then treated with dimethyl aniline gives a coloured product. Its structure would be: [AIPMT (S) 2004]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
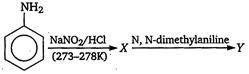
question_answer 12)
Aniline in a set of reactions yielded a product D. [AIPMT (S) 2005] The structure of die product D would be:
A)
C6 H5 CH2 > H2
done
clear
B)
C6H5 NHCH2CH3
done
clear
C)
C6H5 NHOH
done
clear
D)
C6H5 CH2 OH
done
clear
View Answer play_arrow
question_answer 13) . Electrolytic reduction of nitrobenzene in weakly acidic medium gives: [AIPMT (S) 2005]
A)
aniline
done
clear
B)
nitrosobenzene
done
clear
C)
N-phenylhydroxylamine
done
clear
D)
p-hydroxyaniline
done
clear
View Answer play_arrow
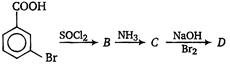
question_answer 14)
In a set of reactions propionic acid yielded a compound D. [AIPMT (S) 2006] \[C{{H}_{3}}C{{H}_{2}}COO\xrightarrow[{}]{SOC{{l}_{2}}}B\xrightarrow[{}]{N{{H}_{3}}}C\xrightarrow[B{{r}_{2}}]{KOH}D\] The structure of D would be:
A)
CH3CH2CH2NH2
done
clear
B)
CH3CH2CONH2
done
clear
C)
CH3CH2NHCH3
done
clear
D)
CH3CH2NH2
done
clear
View Answer play_arrow
question_answer 15) Which of the following is more basic than aniline? [AIPMT (S) 2006]
A)
Diphenylamine
done
clear
B)
Triphenylamine
done
clear
C)
p-nitroaniline
done
clear
D)
Benzyiamine
done
clear
View Answer play_arrow
question_answer 16) Which one of the following on reduction with lithium aluminium hydride yield a secondary amine?[AIPMT (S) 2007]
A)
Nitroethane
done
clear
B)
Methylisocyanide
done
clear
C)
Acetamide
done
clear
D)
Methyl cyanide
done
clear
View Answer play_arrow
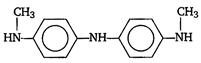
question_answer 17)
In a reaction of aniline a coloured product C was obtained. [AIPMT (S) 2008] The structure of C would be
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 18)
Predict the product, [AIPMT (S) 2009]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 19)
Aniline in a set of the following reactions yielded a coloured product V. [AIPMT (S) 2010] The structure of 'Y' would be
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 20) Which of the following statements about primary amines is false? [AIPMT (S) 2010]
A)
Alkyl amines are stronger bases than aryl amines
done
clear
B)
Alkyl amines react with nitrous acid to produce alcohols
done
clear
C)
Aryl amines react with nitrous acid to produce phenols
done
clear
D)
Alkyl amines are stronger bases than ammonia
done
clear
View Answer play_arrow
question_answer 21)
Match the compounds given in List I with their characteristic reactions given in List II. Select the correct option. [AIPMT (M) 2010] List I (Compounds) List II (Reactions) A.\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\]\[\text{C}{{\text{H}}_{\text{2}}}\text{N}{{\text{H}}_{\text{2}}}\] B.\[\text{C}{{\text{H}}_{3}}\text{C}\equiv \text{CH}\] C. \[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{COOC}{{\text{H}}_{\text{3}}}\] D.\[\text{C}{{\text{H}}_{\text{3}}}\text{CH(OH)C}{{\text{H}}_{\text{3}}}\] 1. Alkaline hydrolysis 2. With KOH (alcohol) And \[\text{CHC}{{\text{l}}_{\text{3}}}\]produces bad smell 3. Gives white ppt with ammoniacal \[\text{AgN}{{\text{O}}_{\text{3}}}\] 4. With Lucas reagent cloudiness appears after 5 min
A)
A-2, B-1, C-4, D-3
done
clear
B)
A-3, B-2, C-1, D-4
done
clear
C)
A-2, B-3, C-1, D-4
done
clear
D)
A-4, B-2, C-3, D-1
done
clear
View Answer play_arrow
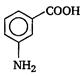
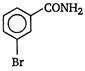
question_answer 22)
In a set of reactions, m-bromobenzoic acid gave a product D. Identify the product D. [AIPMT (S) 2011]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 23)
What is the product obtained in the following reaction? [AIPMT (S) 2011]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 24) Which of the following compound is most basic? [AIPMT (M) 2011]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
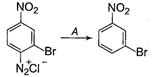
question_answer 25)
In the reaction, A is [NEET 2013]
A)
\[HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{u}_{2}}C{{l}_{2}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{2}}\]and\[{{H}_{2}}O\]
done
clear
D)
\[{{H}^{+}}/{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 26)
In the following reaction, the product is [AIPMT 2014]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 27) The electrolytic reduction of nitrobenzene in strongly acidic medium produces [NEET 2015 ]
A)
p-aminophenol
done
clear
B)
azoxybenzene
done
clear
C)
azobenzene
done
clear
D)
aniline
done
clear
View Answer play_arrow
question_answer 28) Method by which aniline cannot be prepared is [NEET 2015 (Re)]
A)
hydrolysis phenyl isocyanide with acidic solution
done
clear
B)
degradation of benzamide with bromine in alkaline solution
done
clear
C)
reduction of nitrobenzene with \[{{H}_{2}}/Pd\] in ethanol
done
clear
D)
potassium salt of phthalimide treated with chlorobenzene followed by the hydrolysis with aqueous \[NaOH\] solution
done
clear
View Answer play_arrow
question_answer 29) The correct statement regarding the basicity of arylamines is :- [NEET - 2016]
A)
Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring electron system.
done
clear
B)
Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring electron system.
done
clear
C)
Arylamines are generally more basic than alkylamines because of aryl group.
done
clear
D)
Arylamines are generally more basic than alkylamines, because the nitrongen atom in arylamines is sp-hybridized.
done
clear
View Answer play_arrow
question_answer 30) Which one given below is a non-reducing sugar? [NEET - 2016]
A)
Maltose
done
clear
B)
Lactose
done
clear
C)
Glucose
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 31) Which of the following reactions is zappropriate for converting acetamide to methanamine? [NEET-2017]
A)
Gabriels phthalimide synthesis
done
clear
B)
Carbylamine reaction
done
clear
C)
Hoffmann hypobromamide reaction
done
clear
D)
Stephens reaction
done
clear
View Answer play_arrow
question_answer 32) Nitration of aniline in strong acidic medium also gives m-nitroaniline because [NEET - 2018]
A)
In absence of substituents nitro group always goes to m-position.
done
clear
B)
In electrophilic substitution reactions amino group is meta directive.
done
clear
C)
Inspite of substituents nitro group always goes to only m-position.
done
clear
D)
In acidic (strong) medium aniline is present as anilinium ion.
done
clear
View Answer play_arrow
question_answer 33) The correct order of the basic strength of methyl substituted amines in aqueous solution is- [NEET 2019]
A)
\[{{(C{{H}_{3}})}_{3}}N.{{\text{(}C{{H}_{3}}\text{)}}_{2}}NH>C{{H}_{3}}N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}N{{H}_{2}}>{{\text{(}C{{H}_{3}}\text{)}}_{2}}NH>{{\text{(}C{{H}_{3}}\text{)}}_{3}}N\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}NH>C{{H}_{3}}N{{H}_{2}}>{{\text{(}C{{H}_{3}}\text{)}}_{3}}N\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}N>C{{H}_{3}}N{{H}_{2}}>{{\text{(}C{{H}_{3}}\text{)}}_{2}}NH\]
done
clear
View Answer play_arrow
question_answer 34)
The major product of the following reaction is: [NEET 2019]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 35) Which of the following amine will give the carbylamine test? [NEET 2020]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
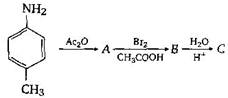 would be:
would be: