-
question_answer1) In a face centered cubic lattice atoms A are at the corner points and atoms B at the face centered points. If atom B is missing from one of the face centered points, the formula of the ionic compound is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[A{{B}_{2}}\]
done
clear
B)
\[{{A}_{5}}{{B}_{2}}\]
done
clear
C)
\[{{A}_{2}}{{B}_{3}}\]
done
clear
D)
\[{{A}_{2}}{{B}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer2) Van der Waal's equation for a gas is stated as, \[p=\frac{nRT}{V-nb}-a{{\left( \frac{n}{V} \right)}^{2}}.\] This equation reduces to the perfect gas equation,\[p=\frac{nRT}{V}\]when,
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
temperature is sufficient high and pressure is low.
done
clear
B)
temperature is sufficient low and pressure is high.
done
clear
C)
both temperature and pressure are very high.
done
clear
D)
both temperature and pressure are very low.
done
clear
View Answer play_arrow
-
question_answer3) The standard electrode potentials \[\left( E_{{{M}^{+}}/M}^{0} \right)\] of four metals A, B, C and D are - 1.2 V, 0.6 V, 0.85 V and - 0.76 V, respectively. The sequence of deposition of metals on applying potential is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
A, C, B, D
done
clear
B)
B, D, C, A
done
clear
C)
C, B, D, A
done
clear
D)
D, A, B, C
done
clear
View Answer play_arrow
-
question_answer4) At a certain temperature, only 50% HI is dissociated into \[{{H}_{2}}\]and \[{{I}_{2}}\] at equilibrium. The equilibrium constant is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1.0
done
clear
B)
3.0
done
clear
C)
0.5
done
clear
D)
0.25
done
clear
View Answer play_arrow
-
question_answer5) Dissolving 120 g of a compound of (mol. wt. 60) in 1000 g of water gave a solution of density 1.12 g/mL. The molarity of the solution is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1.00M
done
clear
B)
2.00 M
done
clear
C)
2.50 M
done
clear
D)
4.00 M
done
clear
View Answer play_arrow
-
question_answer6) The half-life period of a first order reaction is 15 minutes. The amount of substance left after one hour will be:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[\frac{1}{4}\]of the original amount
done
clear
B)
\[\frac{1}{8}\]of the original amount
done
clear
C)
\[\frac{1}{16}\]of the original amount
done
clear
D)
\[\frac{1}{32}\]of the original amount
done
clear
View Answer play_arrow
-
question_answer7) A current of 10.0 A flows for 2.00 h through an electrolytic cell containing a molten salt of metal X. This results in the decomposition of 0.250 mol of metal X at the cathode. The oxidation state of X in the molten salt is: (F = 96,500 C)
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
1+
done
clear
B)
2 +
done
clear
C)
3+
done
clear
D)
4 +
done
clear
View Answer play_arrow
-
question_answer8) The energy of an electron in first Bohr orbit of H-atom is -13.6 eV. The energy value of electron in the excited state of\[L{{i}^{2+}}\]is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
27.2 eV
done
clear
B)
30.6 eV
done
clear
C)
30.6 eV
done
clear
D)
27.2 eV
done
clear
View Answer play_arrow
-
question_answer9) The temperature at which oxygen molecules have the same root mean square speed as helium atoms have at 300 K is: Atomic masses: He = 4 u, O = 16 u)
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
300 K
done
clear
B)
600 K
done
clear
C)
1200 K
done
clear
D)
2400 K
done
clear
View Answer play_arrow
-
question_answer10) The standard enthalpy of formation of \[N{{H}_{3}}\] is -46.0 kJ/mol. If the enthalpy of formation of \[{{H}_{2}}\] from its atoms is -436 kJ/mol and that of \[{{N}_{2}}\] is - 712 kJ/mol, the average bond enthalpy of N - H bond in \[N{{H}_{3}}\] is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
-1102 kJ/mol
done
clear
B)
- 964 kJ/mol
done
clear
C)
+ 352 kJ/mol
done
clear
D)
+ 1056 kJ/mol
done
clear
View Answer play_arrow
-
question_answer11) The amount of oxygen in 3.6 moles of water is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
115.2 g
done
clear
B)
57.6 g
done
clear
C)
28.8 g
done
clear
D)
18.4 g
done
clear
View Answer play_arrow
-
question_answer12) The gas evolved on heating \[Ca{{F}_{2}}\]and \[Si{{O}_{2}}\] with concentrated\[{{H}_{2}}S{{O}_{4}},\]on hydrolysis gives a white gelatinous precipitate. The precipitate is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Hydro fluosilicic acid
done
clear
B)
silica gel
done
clear
C)
silicic acid
done
clear
D)
calcium fluorosilicate
done
clear
View Answer play_arrow
-
question_answer13) Chloro compound of Vanadium has only spin magnetic moment of 1.73 BM. This Vanadium chloride has the formula:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[VC{{l}_{2}}\]
done
clear
B)
\[VC{{l}_{4}}\]
done
clear
C)
\[VC{{l}_{3}}\]
done
clear
D)
\[VC{{l}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer14) An octahedral complex of \[C{{o}^{3+}}\]is diamagnetic. The hybridisation involved in the formation of the complex is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
B)
\[ds{{p}^{2}}\]
done
clear
C)
\[{{d}^{2}}s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}}d\]
done
clear
View Answer play_arrow
-
question_answer15) Which of the following is not formed when \[{{H}_{2}}S\] reacts with acidic \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]solution?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[CrS{{O}_{4}}\]
done
clear
B)
\[C{{r}_{2}}{{(S{{O}_{4}})}_{3}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
D)
\[S\]
done
clear
View Answer play_arrow
-
question_answer16) Which of the following has unpaired electron(s)?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{N}_{2}}\]
done
clear
B)
\[O_{2}^{-}\]
done
clear
C)
\[N_{2}^{2+}\]
done
clear
D)
\[N_{2}^{2-}\]
done
clear
View Answer play_arrow
-
question_answer17)
In the following sets of reactants which two sets best exhibit the amphoteric characters of \[A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O\]?
|
Set \[1:A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O(s)\]and \[O{{H}^{-}}(aq)\]
|
|
Set \[2:A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O(s)\]and \[{{H}_{2}}O(l)\]
|
|
Set \[3:A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O(s)\]and \[{{H}^{+}}(aq)\]
|
|
Set \[4:A{{l}_{2}}{{O}_{3}}.x{{H}_{2}}O(s)\]and \[N{{H}_{3}}(aq)\]
|
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[1 and 2\]
done
clear
B)
\[1 and 3\]
done
clear
C)
\[2 and 4\]
done
clear
D)
\[3 and 4\]
done
clear
View Answer play_arrow
-
question_answer18) The number and type of bonds in \[C_{2}^{2-}\]ion in \[Ca{{C}_{2}}\] are:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
One \[\sigma \] bond and one \[\text{ }\!\!\pi\!\!\text{ -}\]bond
done
clear
B)
One \[\sigma \] bond and two \[\text{ }\!\!\pi\!\!\text{ -}\]bond
done
clear
C)
Two \[\sigma \] bond and two \[\text{ }\!\!\pi\!\!\text{ -}\]bond
done
clear
D)
Two \[\sigma \] bond and one \[\text{ }\!\!\pi\!\!\text{ -}\]bond
done
clear
View Answer play_arrow
-
question_answer19) The form of iron obtained from blast furnace is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Steel
done
clear
B)
Cast Iron
done
clear
C)
Pig Iron
done
clear
D)
Wrought Iron
done
clear
View Answer play_arrow
-
question_answer20) Which one of the following reactions will not result in the formation of carbon-carbon bond?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Reimer-Tie man reaction
done
clear
B)
Friedel Craft's acylation
done
clear
C)
Wurtz reaction
done
clear
D)
Cannizzaro reaction
done
clear
View Answer play_arrow
-
question_answer21) In the hydrocarboration - oxidation reaction of propene with diborane, \[{{H}_{2}}{{O}_{2}}\]and NaOH, the organic compound formed is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}CHOHC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
View Answer play_arrow
-
question_answer22)
The major product of the reaction 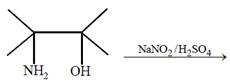 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer23) For the compounds\[C{{H}_{3}}Cl,C{{H}_{3}}Br,C{{H}_{3}}I\]and \[C{{H}_{3}}F,\]the correct order of increasing C-halogen bond length is:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[C{{H}_{3}}F<C{{H}_{3}}Cl<C{{H}_{3}}Br<C{{H}_{3}}I\]
done
clear
B)
\[C{{H}_{3}}F<C{{H}_{3}}Br<C{{H}_{3}}CI<C{{H}_{3}}I\]
done
clear
C)
\[C{{H}_{3}}F<C{{H}_{3}}I<C{{H}_{3}}Br<C{{H}_{3}}Cl\]
done
clear
D)
\[C{{H}_{3}}Cl<C{{H}_{3}}Br<C{{H}_{3}}F<C{{H}_{3}}I\]
done
clear
View Answer play_arrow
-
question_answer24) Allyl phenyl ether can be prepared by heating:
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{C}_{6}}{{H}_{5}}Br+C{{H}_{2}}=CH-C{{H}_{2}}-ONa\]
done
clear
B)
\[C{{H}_{2}}=CH-C{{H}_{2}}-Br-{{C}_{6}}{{H}_{5}}ONa\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}-CH=CH-Br-C{{H}_{3}}-ONa\]
done
clear
D)
\[C{{H}_{2}}=CH-Br-{{C}_{6}}{{H}_{5}}-C{{H}_{2}}-ONa\]
done
clear
View Answer play_arrow
-
question_answer25) In a nucleophilic substitution reaction: \[R-Br+C{{l}^{-}}\xrightarrow[{}]{DMF}R-Cl+B{{r}^{-}},\]which one of the following undergoes complete inversion of configuration?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[{{C}_{6}}{{H}_{5}}CH{{C}_{6}}{{H}_{5}}Br\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}Br\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CHC{{H}_{3}}Br\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}CC{{H}_{3}}{{C}_{6}}{{H}_{5}}Br\]
done
clear
View Answer play_arrow
-
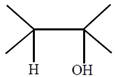
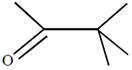
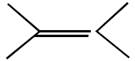
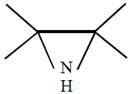
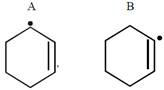
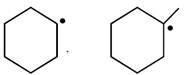
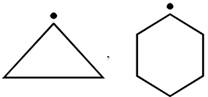
question_answer26) In which of the following pairs A is more stable than B?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
done
clear
B)
done
clear
C)
done
clear
D)
\[P{{h}_{3}}{{C}^{\bullet }},{{(C{{H}_{3}})}_{3}}{{C}^{\bullet }}\]
done
clear
View Answer play_arrow
-
question_answer27) Structure of some important polymers are given. Which one represents Buna-S?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
\[(-C{{H}_{2}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,=CH-C{{H}_{2}}-)n\]
done
clear
B)
\[(-C{{H}_{2}}-CH=CH-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ {{C}_{6}}{{H}_{5}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}\overline{n})\]
done
clear
C)
\[(-C{{H}_{2}}-CH=CH-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ CN \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-)n\]
done
clear
D)
\[{{(-C{{H}_{2}}-\overset{\begin{smallmatrix} Cl \\ | \end{smallmatrix}}{\mathop{C}}\,=CH-C{{H}_{2}}-)}_{n}}\]
done
clear
View Answer play_arrow
-
question_answer28) Which is major product formed when acetone is heated with iodine and potassium hydroxide?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Iodoacetone
done
clear
B)
Acetic acid
done
clear
C)
Iodoform
done
clear
D)
Acetophenone
done
clear
View Answer play_arrow
-
question_answer29) Which one of the following class of compounds is obtained by polymerization of acetylene?
[JEE Main Online Paper ( Held On 09 Apirl 2014 )
A)
Poly-yne
done
clear
B)
Poly-ene
done
clear
C)
Poly-ester
done
clear
D)
Poly-amine
done
clear
View Answer play_arrow
 [JEE Main Online Paper ( Held On 09 Apirl 2014 )
[JEE Main Online Paper ( Held On 09 Apirl 2014 )