-
question_answer1) If \[{{\lambda }_{o}}\]and \[\lambda \] be threshold wavelength and wavelength of incident light, the velocity of photoelectron ejected from the metal surface is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[\sqrt{\frac{2h}{m}\left( {{\lambda }_{o}}-\lambda \right)}\]
done
clear
B)
\[\sqrt{\frac{2hc}{m}\left( {{\lambda }_{o}}-\lambda \right)}\]
done
clear
C)
\[\sqrt{\frac{2hc}{m}\left( \frac{{{\lambda }_{o}}-\lambda }{\lambda {{\lambda }_{o}}} \right)}\]
done
clear
D)
\[\sqrt{\frac{2h}{m}\left( \frac{1}{{{\lambda }_{o}}}-\frac{1}{\lambda } \right)}\]
done
clear
View Answer play_arrow
-
question_answer2) The appearance of colour in solid alkali meta halides is generally due to:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Schottky defect
done
clear
B)
Frenkel defect
done
clear
C)
Interstitial position
done
clear
D)
F-centres
done
clear
View Answer play_arrow
-
question_answer3) In the reaction of formation of sulphur trioxide by contact process \[2S{{O}_{2}}+{{O}_{2}}\rightleftharpoons 2S{{O}_{3}}\]the rate of reaction was measured as\[\frac{d\left[ {{O}_{2}} \right]}{dt}=-2.5\times {{10}^{-4}}\text{mol}\]\[\,{{L}^{-1}}{{s}^{-1}}.\] The rate of reaction is terms of \[[S{{O}_{2}}]\]in mol \[{{\text{L}}^{\text{-1}}}\,{{\text{s}}^{\text{-1}}}\] will be:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[-1.25\times {{10}^{-4}}\]
done
clear
B)
\[-2.50\times {{10}^{-4}}\]
done
clear
C)
\[-3.75\times {{10}^{-4}}\]
done
clear
D)
\[-5.00\times {{10}^{-4}}\]
done
clear
View Answer play_arrow
-
question_answer4) Assuming that the degree of hydrolysis is small, the pH of 0.1 M solution of sodium acetate \[({{K}_{a}}=1.0\times {{10}^{-5}})\] will be:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
5.0
done
clear
B)
6.0
done
clear
C)
8.0
done
clear
D)
9.0
done
clear
View Answer play_arrow
-
question_answer5) For the reaction, \[2{{N}_{2}}{{O}_{5}}\to 4N{{O}_{2}}+{{O}_{2}},\]the rate equation can be expressed in two ways\[-\frac{d\left[ {{N}_{2}}{{O}_{5}} \right]}{dt}=k\left[ {{N}_{2}}{{O}_{5}} \right]\] and \[+\frac{d\left[ N{{O}_{2}} \right]}{dt}=k'\left[ {{N}_{2}}{{O}_{5}} \right]\]k and k' are related as:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
k = k'
done
clear
B)
2k = k'
done
clear
C)
k = 2k'
done
clear
D)
k = 4k'
done
clear
View Answer play_arrow
-
question_answer6) In some solutions, the concentration of \[{{H}_{3}}{{O}^{+}}\] remains constant even when small amounts of strong acid or strong base are added to them. These solutions are known as:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Ideal solutions
done
clear
B)
Colloidal solutions
done
clear
C)
True solutions
done
clear
D)
Buffer solutions
done
clear
View Answer play_arrow
-
question_answer7) Given \[F{{e}^{3+}}(aq)+{{e}^{-}}\to F{{e}^{2+}}(aq);{{E}^{0}}=+0.77V\] \[A{{l}^{3+}}(aq)+3{{e}^{-}}\to Al(s);{{E}^{0}}=-1.66V\] \[B{{r}_{2}}(aq)+2{{e}^{-}}\to 2B{{r}^{-}};{{E}^{0}}=+1.09V\] Considering the electrode potentials, which of the following represents the correct order of reducing power?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[F{{e}^{2+}}<Al<B{{r}^{-}}\]
done
clear
B)
\[B{{r}^{-}}<F{{e}^{2+}}<Al\]
done
clear
C)
\[Al<B{{r}^{-}}<F{{e}^{2+}}\]
done
clear
D)
\[Al<F{{e}^{2+}}<B{{r}^{-}}\]
done
clear
View Answer play_arrow
-
question_answer8) The initial volume of a gas cylinder is 750.0 mL. If the pressure of gas inside the cylinder changes from 840.0 mm Hg to 360.0 mm Hg, the final volume the gas will be:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
1.750 L
done
clear
B)
3.60 L
done
clear
C)
4.032 L
done
clear
D)
7.50 L
done
clear
View Answer play_arrow
-
question_answer9) The molar heat capacity \[({{C}_{p}})\] of \[C{{D}_{2}}O\]is 10 cals at 1000 K. The change in entropy associated with cooling of 32 g of \[C{{D}_{2}}O\]vapour from 1000 K to 100 K at constant pressure will be: (D = deuterium, atomic mass = 2 u)
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[\text{23}\text{.03cal}\,\text{de}{{\text{g}}^{\text{-1}}}\]
done
clear
B)
\[\text{-23}\text{.03cal}\,\text{de}{{\text{g}}^{\text{-1}}}\]
done
clear
C)
\[\text{2}\text{.303cal}\,\text{de}{{\text{g}}^{\text{-1}}}\]
done
clear
D)
\[\text{-2}\text{.303cal}\,\text{de}{{\text{g}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
-
question_answer10) Based on the equation: \[\Delta E=-2.0\times {{10}^{-18}}J\left( \frac{1}{n_{2}^{2}}-\frac{1}{n_{1}^{2}} \right)\]the wavelength of the light that must be absorbed to excite hydrogen electron from level n = 1 to level n = 2 will be: \[(h=6.625\times {{10}^{-34}}Js,C=3\times {{10}^{8}}m{{s}^{-1}})\]
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[1.325\times {{10}^{-7}}m\]
done
clear
B)
\[1.325\times {{10}^{-10}}m\]
done
clear
C)
\[2.650\times {{10}^{-7}}m\]
done
clear
D)
\[5.300\times {{10}^{-10}}m\]
done
clear
View Answer play_arrow
-
question_answer11) Which of the following series correctly represents relations between the elements from X to Y? \[X\to Y\]
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[_{3}Li{{\to }_{19}}K\] Ionization enthalpy increases
done
clear
B)
\[_{9}F{{\to }_{35}}Br\] Electron gain enthalpy (negative sign) increases
done
clear
C)
\[_{6}C{{\to }_{32}}Ge\] Atomic radii increases
done
clear
D)
\[_{18}Ar{{\to }_{54}}Xe\] Noble character increases
done
clear
View Answer play_arrow
-
question_answer12) The correct order of bond dissociation energy among \[{{N}_{2}},{{O}_{2}},O_{2}^{-}\]is shown in which of the following arrangements?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[{{N}_{2}}>O_{2}^{-}>{{O}_{2}}\]
done
clear
B)
\[O_{2}^{-}>{{O}_{2}}>{{N}_{2}}\]
done
clear
C)
\[N_{2}^{{}}>{{O}_{2}}>O_{2}^{-}\]
done
clear
D)
\[{{O}_{2}}>O_{2}^{-}>N_{2}^{{}}\]
done
clear
View Answer play_arrow
-
question_answer13) Which of the following statements about \[N{{a}_{2}}{{O}_{2}}\] is not correct?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
It is diamagnetic in nature
done
clear
B)
It is derivative of \[{{H}_{2}}{{O}_{2}}\]
done
clear
C)
\[N{{a}_{2}}{{O}_{2}}\]oxidises \[C{{r}^{3+}}\] to \[CrO_{4}^{2-}\]in acid medium.
done
clear
D)
It is the super oxide of sodium
done
clear
View Answer play_arrow
-
question_answer14) Which of the following statements about the depletion of ozone layer is correct?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
The problem of ozone depletion is less serious at poles because \[N{{O}_{2}}\] solidifies and is not available for consuming \[CI{{O}^{\bullet }}\]radicals.
done
clear
B)
The problem of ozone depletion is more serious at poles because ice crystals in the clouds over poles act as catalyst for photochemical reactions involving the decomposition of ozone of \[C{{l}^{\bullet }}\] and \[Cl{{O}^{\bullet }}\] radicals.
done
clear
C)
Freons, chlorofluorocarbons, are inert. Chemically, they do not react with ozone in stratosphere.
done
clear
D)
Oxides of nitrogen also do not react with ozone in stratosphere.
done
clear
View Answer play_arrow
-
question_answer15) A gaseous compound of nitrogen and hydrogen contains 12.5% (by mass) of hydrogen. The density of the compound relative to hydrogen is 16. The molecular formula of the compound is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[N{{H}_{2}}\]
done
clear
B)
\[{{N}_{3}}H\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[{{N}_{2}}{{H}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer16) Shapes of certain interhalogen compounds are stated below. Which one of them is not correctly stated?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[\text{I}{{\text{F}}_{\text{7}}}\] : pentagonal bipyramid
done
clear
B)
\[\text{Br}{{\text{F}}_{5}}\] : trigonal bipyramid
done
clear
C)
\[\text{Br}{{\text{F}}_{3}}\] : planar T-shaped
done
clear
D)
\[\text{IC}{{\text{I}}_{3}}\]: planar dimeric
done
clear
View Answer play_arrow
-
question_answer17) Consider the following equilibrium \[AgCl\downarrow +2N{{H}_{3}}\rightleftharpoons {{\left[ Ag{{\left( N{{H}_{3}} \right)}_{2}} \right]}^{+}}+C{{l}^{-}}\] White precipitate of AgCl appears on adding which of the following?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[N{{H}_{3}}\] aqueous NaCl
done
clear
B)
aqueous \[HN{{O}_{3}}\]
done
clear
C)
aqueous \[N{{H}_{4}}Cl\]
done
clear
View Answer play_arrow
-
question_answer18) Which of the following name formula combinations is not correct?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
| Formula |
|
| \[{{K}_{2}}[Pt{{\left( CN \right)}_{4}}]\] |
Potassium tetracyanoplatinate (II) |
done
clear
B)
| Formula |
|
| \[{{[Mn\left( CN \right)5]}^{2}}\] |
Pentacyanomagnate (II) ion |
done
clear
C)
| Formula |
|
| \[K[Cr{{(N{{H}_{3}})}_{2}}C{{l}_{4}}]\] |
Potassium diammine tetrachlorochromate (III) |
done
clear
D)
| Formula |
|
| \[[Co{{(N{{H}_{3}})}_{4}}({{H}_{2}}O)I]S{{O}_{4}}\] |
Magnetron value. |
done
clear
View Answer play_arrow
-
question_answer19) Consider the coordination compound, \[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}.\]In the formation of this complex, the species which acts as the Lewis acid is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
B)
\[C{{l}^{-}}\]
done
clear
C)
\[C{{o}^{3+}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer20) Which one of the following does not have a pyramidal shape?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[{{(C{{H}_{3}})}_{3}}N\]
done
clear
B)
\[{{(Si{{H}_{3}})}_{3}}N\]
done
clear
C)
\[P{{(C{{H}_{3}})}_{3}}\]
done
clear
D)
\[P{{(Si{{H}_{3}})}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer21)
The following reaction 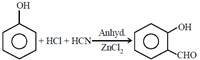 is known as:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
is known as:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Perkin reaction
done
clear
B)
Gatterman-Koch Formylation
done
clear
C)
Kolbe?s reaction
done
clear
D)
Gattermann reaction
done
clear
View Answer play_arrow
-
question_answer22)
The reagent needed for converting 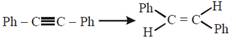 [JEE Main Online Paper ( Held On 11 Apirl 2014 )
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Cat. Hydrogenation
done
clear
B)
\[{{H}_{2}}/\]Lindlar Cat.
done
clear
C)
\[Li/N{{H}_{3}}\]
done
clear
D)
\[LiAl{{H}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer23) Complete reduction of benzene-diazonium chloride with Zn/ HCl gives:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Aniline
done
clear
B)
Phenylhydrazine
done
clear
C)
Azobenzene
done
clear
D)
Hydrazobenzene
done
clear
View Answer play_arrow
-
question_answer24)
An organic compound \[A,{{C}_{5}}{{H}_{8}}O;\]reacts with \[{{H}_{2}}O,N{{H}_{3}}\]and \[C{{H}_{3}}COOH\] as described below: 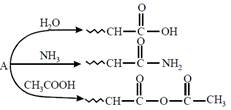 A is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
\[C{{H}_{3}}CH=\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-CHO\]
done
clear
B)
\[C{{H}_{2}}=CH\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-CHO\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,=C=O\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ C{{H}_{2}} \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\mathop{C}}\,=O\]
done
clear
View Answer play_arrow
-
question_answer25) In allene \[({{C}_{3}}{{H}_{4}}),\]the type(s) of hybridization of the carbon atoms is (are) :
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
sp and \[\text{s}{{\text{p}}^{\text{3}}}\]
done
clear
B)
\[\text{s}{{\text{p}}^{2}}\]and sp
done
clear
C)
only
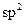
D)
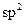
and
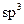
done
clear
View Answer play_arrow
-
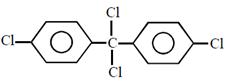
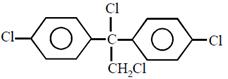
question_answer26)
Chlorobenzne reacts with trichloro acetaldehyde in the presence of 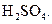
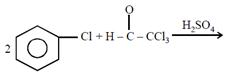 The major product formed is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
The major product formed is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer27) Tischenko reaction is a modification of:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Aldol condensation
done
clear
B)
Claisen condensation
done
clear
C)
Cannizzaro reaction
done
clear
D)
Pinacol-pinacolon reaction
done
clear
View Answer play_arrow
-
question_answer28) Which one of the following is used as Antihistamine?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Omeprazole
done
clear
B)
Chloranphenicol
done
clear
C)
Diphenhydramine
done
clear
D)
Norethindrone
done
clear
View Answer play_arrow
-
question_answer29) Which one of the following statements is not correct?
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Alcohols are weaker acids than water
done
clear
B)
Acid strength of alcohols decreases in the following \[RC{{H}_{2}}>{{R}_{2}}CHOH>{{R}_{3}}COH\]
done
clear
C)
Carbon-oxygen bond length in methanol, \[C{{H}_{3}}OH\] is shorter than that of C - O bond length in phenol.
done
clear
D)
The bond angle
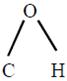
in methanol is \[108.9{}^\circ \].
-
question_answer30) The gas liberated by the electrolysis of Dipotassium succinate solution is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A)
Ethane
done
clear
B)
Ethyne
done
clear
C)
Ethene
done
clear
D)
PropeneAT
done
clear
View Answer play_arrow
 is known as:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
is known as:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
 A is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
A is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
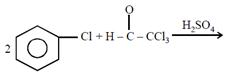 The major product formed is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
The major product formed is:
[JEE Main Online Paper ( Held On 11 Apirl 2014 )
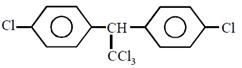
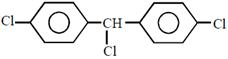
 in methanol is \[108.9{}^\circ \].
in methanol is \[108.9{}^\circ \]. 