-
question_answer1)
The following sets of quantum numbers represent four electrons in an atom.
JEE Main Online Paper (Held On 26-May-2012)
|
(i) \[n=4,l=1\]
|
|
(ii) \[n=4,l=0\]
|
|
(iii)\[n=3,l=2\]
|
|
(iv) \[n=3,l=1\]
|
The sequence representing increasing order of energy, is
A)
(iii)<(i)<(iv)<(ii)
done
clear
B)
(iv)<(ii)<(iii)<(i)
done
clear
C)
(i)<(iii)<(ii)<(iv)
done
clear
D)
(ii)<(iv)<(i)<(iii)
done
clear
View Answer play_arrow
-
question_answer2) 'The substance used as froth stabilisers in forth-floatation process is .
JEE Main Online Paper (Held On 26-May-2012)
A)
Potassium ethyl xanthate
done
clear
B)
Aniline
done
clear
C)
Sodium cyanide
done
clear
D)
Copper sulphate
done
clear
View Answer play_arrow
-
question_answer3) The activation energy for a reaction which doubles the rate when the temperature is raised from 298 K to 308 K is.
JEE Main Online Paper (Held On 26-May-2012)
A)
\[52.2\,kJ\,mo{{l}^{-1}}\]
done
clear
B)
\[39.2\,kJ\,mo{{l}^{-1}}\]
done
clear
C)
\[52.9\,kJ\,mo{{l}^{-1}}\]
done
clear
D)
\[29.5\,kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer4) Tollen's reagent and Fehling solutions are used to distinguish between.
JEE Main Online Paper (Held On 26-May-2012)
A)
acids and alcohols
done
clear
B)
alkanes and alcohols
done
clear
C)
ketones and aldehydes
done
clear
D)
n-alkaens and branched alkanes
done
clear
View Answer play_arrow
-
question_answer5)
In the following compounds : 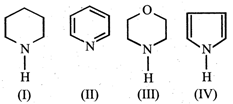 the order of basicity is as follows.
JEE Main Online Paper (Held On 26-May-2012)
the order of basicity is as follows.
JEE Main Online Paper (Held On 26-May-2012)
A)
\[IV>III>II>I\]
done
clear
B)
\[III>I>II>IV\]
done
clear
C)
\[II>III>I>IV\]
done
clear
D)
\[I>III>II>IV\]
done
clear
View Answer play_arrow
-
question_answer6) Maleic acid and fumaric acids are.
JEE Main Online Paper (Held On 26-May-2012)
A)
Chain isomers
done
clear
B)
Functional isomers
done
clear
C)
Tautomers
done
clear
D)
Geometrical isomers
done
clear
View Answer play_arrow
-
question_answer7)
Which of the following compounds are anti-aromatic 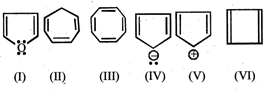 .
JEE Main Online Paper (Held On 26-May-2012)
.
JEE Main Online Paper (Held On 26-May-2012)
A)
(I) and (V)
done
clear
B)
(II) and (V)
done
clear
C)
(I) and (IV)
done
clear
D)
(III) and (VI)
done
clear
View Answer play_arrow
-
question_answer8) The freezing point of a 1.00 m aqueous solution of HF is found to be \[-1.91\text{ }{}^\circ C\]. The freezing point constant of water, K. is 1.86 K kg \[\text{mo}{{\text{l}}^{-1}}\] The percentage dissociation of HF at this concentration is.
JEE Main Online Paper (Held On 26-May-2012)
A)
30%
done
clear
B)
10%
done
clear
C)
5.2%
done
clear
D)
2.7%
done
clear
View Answer play_arrow
-
question_answer9) Which of the following presents the correct order of second ionization enthalpies of C, N, O and F?
JEE Main Online Paper (Held On 26-May-2012)
A)
\[F>O>N>C\]
done
clear
B)
\[O>N>F>C\]
done
clear
C)
\[C>N>O>F\]
done
clear
D)
\[O>F>N>C\]
done
clear
View Answer play_arrow
-
question_answer10) A transition metal M forms a volatile chloride which has a vapour density of 94.8. If it contains74.75% of chlorine the formula of the metal chloride will be .
JEE Main Online Paper (Held On 26-May-2012)
A)
\[MC{{l}_{3}}\]
done
clear
B)
\[MC{{l}_{2}}\]
done
clear
C)
\[MC{{l}_{4}}\]
done
clear
D)
\[MC{{l}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer11)
Among the following species which two have trigonal bipyramidal shape?
JEE Main Online Paper (Held On 26-May-2012)
|
(I) \[N{{I}_{3}}\]
|
|
(II) \[I_{3}^{-}\]
|
|
(III) \[SO_{3}^{2-}\]
|
|
(IV) \[NO_{3}^{-}\]
|
A)
l and III
done
clear
B)
IIl and IV
done
clear
C)
l and IV
done
clear
D)
II and III
done
clear
View Answer play_arrow
-
question_answer12) Consider the following sequence of reactions \[C{{H}_{3}}CH=C{{H}_{2}}\xrightarrow[700K]{C{{l}_{2}}}A\xrightarrow[420K,12atm]{N{{a}_{2}}C{{O}_{3}}}\]\[B\xrightarrow[(ii)NaOH]{(i)HOCl}C\] Compound 'C' is.
JEE Main Online Paper (Held On 26-May-2012)
A)
\[\underset{\begin{align} & | \\ & CHOH \\ & | \\ & C{{H}_{2}}OH \\ \end{align}}{\mathop{C{{H}_{2}}OH}}\,\]
done
clear
B)
\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,COONa\]
done
clear
C)
\[HOC{{H}_{2}}-CH=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,COCl\]
done
clear
View Answer play_arrow
-
question_answer13) Fire extinguishers contain \[{{H}_{2}}S{{O}_{4}}\] and which one of the following?
JEE Main Online Paper (Held On 26-May-2012)
A)
\[NaHC{{O}_{3}}\]and\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
C)
\[NaHC{{O}_{3}}\]
done
clear
D)
\[CaC{{O}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer14) Which one of the following depletes ozone layer?
JEE Main Online Paper (Held On 26-May-2012)
A)
CO
done
clear
B)
NO and freons
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer15) Dipole moment is shown by.
JEE Main Online Paper (Held On 26-May-2012)
A)
1,2-dichlorobenzene
done
clear
B)
trans 2,3-dichloro-2-butene
done
clear
C)
1,4-chlorobenzene
done
clear
D)
trans-1,2-dinitroethene
done
clear
View Answer play_arrow
-
question_answer16) Which of the following statements is correct?
JEE Main Online Paper (Held On 26-May-2012)
A)
RNA controls the synthesis of proteins.
done
clear
B)
The sugar present in DNA is D-(-)-ribose.
done
clear
C)
RNA has double stranded a-helix structure.
done
clear
D)
DNA mainly occurs in the cytoplasm of the cell.
done
clear
View Answer play_arrow
-
question_answer17) The solubility of\[PB{{I}_{2}}\]at 25°C is \[0.7g\,{{L}^{-1}}.\] The solubility product of\[Pb{{I}_{2}}\] at this temperature is(molar mass of\[Pb{{I}_{2}}=461.2\,g\,\text{mo}{{\text{l}}^{-1}}\]).
JEE Main Online Paper (Held On 26-May-2012)
A)
\[1.40\times {{10}^{-9}}\]
done
clear
B)
\[0.14\times {{10}^{-9}}\]
done
clear
C)
\[140\times {{10}^{-9}}\]
done
clear
D)
\[14.0\times {{10}^{-9}}\]
done
clear
View Answer play_arrow
-
question_answer18) Bakelite is obtained from phenol by reacting it with.
JEE Main Online Paper (Held On 26-May-2012)
A)
Acetaldehyde
done
clear
B)
Chlorobenzene
done
clear
C)
Formaldehyde
done
clear
D)
Acetamide
done
clear
View Answer play_arrow
-
question_answer19) Among the following the incorrect statement is.
JEE Main Online Paper (Held On 26-May-2012)
A)
Density of crystals remains unaffected due to Frenkel defect.
done
clear
B)
In BCC unit cell the void space is 32%.
done
clear
C)
Density of crystals decreases due to Schottky defect.
done
clear
D)
Electrical conductivity of semiconductors and metals increases with increase in temperature.
done
clear
View Answer play_arrow
-
question_answer20) Which of the following forms stable + 4 oxidationstate?
JEE Main Online Paper (Held On 26-May-2012)
A)
La(Z=57)
done
clear
B)
Eu(Z=63)
done
clear
C)
Ce(Z=58)
done
clear
D)
Gd(Z=64)
done
clear
View Answer play_arrow
-
question_answer21) Colloidal solutions can be purified by.
JEE Main Online Paper (Held On 26-May-2012)
A)
emulsification
done
clear
B)
electrodialysis
done
clear
C)
peptization
done
clear
D)
using Tyndall effect
done
clear
View Answer play_arrow
-
question_answer22) Given\[E_{C{{u}^{2+}}/Cu}^{o}=0.34V,E_{C{{u}^{2+}}/Cu}^{o}=0.15V\] Standard electrode potential for the half cell \[C{{u}^{+}}/Cu\]is.
JEE Main Online Paper (Held On 26-May-2012)
A)
0.38V
done
clear
B)
0.53V
done
clear
C)
0.19V
done
clear
D)
0.49V
done
clear
View Answer play_arrow
-
question_answer23) The complex ion \[{{[Pt(N{{O}_{2}})(Py)(N{{H}_{3}})(N{{H}_{2}}OH)]}^{+}}\]will give.
JEE Main Online Paper (Held On 26-May-2012)
A)
2 isomers (Geometrical)
done
clear
B)
3 isomers (Geometrical)
done
clear
C)
6 isomers (Geometrical)
done
clear
D)
4 isomers (Geometrical)
done
clear
View Answer play_arrow
-
question_answer24) The hydration of propyne results in formation of.
JEE Main Online Paper (Held On 26-May-2012)
A)
Acetone
done
clear
B)
Propanol-1
done
clear
C)
Propene
done
clear
D)
Propanal
done
clear
View Answer play_arrow
-
question_answer25) One mole of an ideal gas is expanded isothermally and reversibly to half of its initial pressure. \[\Delta S\]for the process in \[J\,{{K}^{-1}}\,mo{{l}^{-1}}\] is [\[\ell n2=0.693\]and\[R=8.314J/(mol\,K)\]].
JEE Main Online Paper (Held On 26-May-2012)
A)
6.76
done
clear
B)
5.76
done
clear
C)
10.76
done
clear
D)
8.03
done
clear
View Answer play_arrow
-
question_answer26) The number of S-S bonds in\[S{{O}_{3}},{{S}_{2}}O_{3}^{2-}{{S}_{2}}O_{6}^{2-}\]and\[{{S}_{2}}O_{8}^{2-}\]respectively are.
JEE Main Online Paper (Held On 26-May-2012)
A)
1,0,0,1
done
clear
B)
1,0,1,0
done
clear
C)
0,1,1,0
done
clear
D)
0,1,0,1
done
clear
View Answer play_arrow
-
question_answer27) Sulphonamides act as.
JEE Main Online Paper (Held On 26-May-2012)
A)
Antiseptic
done
clear
B)
Analgesic
done
clear
C)
Antimicrobials
done
clear
D)
Antipyretic
done
clear
View Answer play_arrow
-
question_answer28) The relationship among most probable velocity, average velocity and root mean square velocity is respectively.
JEE Main Online Paper (Held On 26-May-2012)
A)
\[\sqrt{2}:\sqrt{3}:\sqrt{8/\pi }\]
done
clear
B)
\[\sqrt{2}:\sqrt{8/\pi }:\sqrt{3}\]
done
clear
C)
\[\sqrt{8/\pi }:\sqrt{3}:\sqrt{2}\]
done
clear
D)
\[\sqrt{3}:\sqrt{8/\pi }:\sqrt{2}\]
done
clear
View Answer play_arrow
-
question_answer29) The number of unpaired electrons in Gadolinium\[[Z=64]\]is.
JEE Main Online Paper (Held On 26-May-2012)
A)
3
done
clear
B)
8
done
clear
C)
6
done
clear
D)
2
done
clear
View Answer play_arrow
-
question_answer30) One mole of\[{{O}_{2(g)}}\]and two moles of\[S{{O}_{2(g)}}\] were heated in a closed vessel of one-litre capacity at1098 K. At equilibrium 1.6 moles of\[S{{O}_{3(g)}}\] were found. The equilibrium constant \[{{K}_{c}}\] of the reaction would be.
JEE Main Online Paper (Held On 26-May-2012)
A)
30
done
clear
B)
40
done
clear
C)
80
done
clear
D)
60
done
clear
View Answer play_arrow
 the order of basicity is as follows.
JEE Main Online Paper (Held On 26-May-2012)
the order of basicity is as follows.
JEE Main Online Paper (Held On 26-May-2012)
 .
JEE Main Online Paper (Held On 26-May-2012)
.
JEE Main Online Paper (Held On 26-May-2012)
