-
question_answer1) The artificial sweetener that has the highest sweetness value in comparison to cane sugar is :
JEE Main Online Paper (Held On 09 April 2016)
A)
Saccharin
done
clear
B)
Sucralose
done
clear
C)
Alitame
done
clear
D)
Aspartane
done
clear
View Answer play_arrow
-
question_answer2) The non-metal that does not exhibit positive oxidation state is :
JEE Main Online Paper (Held On 09 April 2016)
A)
Fluorine
done
clear
B)
Oxygen
done
clear
C)
Chlorine
done
clear
D)
Iodine
done
clear
View Answer play_arrow
-
question_answer3)
The reaction of ozone with oxygen atoms in the presence of chlorine atoms can occur by a two step process show below:
|
\[{{O}_{3}}(g)+C{{l}^{\bullet }}(g)\to {{O}_{2}}(g)+Cl{{O}^{\bullet }}(g)\]……(i)
|
|
\[{{k}_{i}}=5.2\times {{10}^{9}}L\,mo{{l}^{-1}}{{s}^{-1}}\]
|
|
\[Cl{{O}^{\bullet }}(g)+{{O}^{\bullet }}(g)\to {{O}_{2}}(g)+C{{l}^{\bullet }}(g)\]……(ii)
|
|
\[{{k}_{ii}}=2.6\times {{10}^{10}}L\,mo{{l}^{-1}}{{s}^{-1}}\]
|
The closest rate constant for the overall reaction\[{{O}_{3}}(g)+{{O}^{\bullet }}(g)\to 2{{O}_{2}}(g)\] is:
JEE Main Online Paper (Held On 09 April 2016)
A)
\[1.4\times {{10}^{20}}L\,mo{{l}^{-1}}\,{{s}^{-1}}\]
done
clear
B)
\[5.2\times {{10}^{9}}L\,mo{{l}^{-1}}\,{{s}^{-1}}\]
done
clear
C)
\[3.1\times {{10}^{10}}L\,mo{{l}^{-1}}\,{{s}^{-1}}\]
done
clear
D)
\[2.6\times {{10}^{10}}L\,mo{{l}^{-1}}\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer4) 5L of an alkane requires 25 L of oxygen for its complete combustion. If all volumes are measured at constant temperature and pressure, the alkane is:
JEE Main Online Paper (Held On 09 April 2016)
A)
Butane
done
clear
B)
Isobutane
done
clear
C)
Ethane
done
clear
D)
Propane
done
clear
View Answer play_arrow
-
question_answer5)
Match the items in Column I with its main use listed in Column II:
| Column I |
Column II |
| (a) Silica gel |
(i) Transistor |
| (b) Silicon |
(ii) Ion-exchanger |
| (c) Silicone |
(iii) Drying agent |
| (d) Silicate |
(iv) Sealant |
JEE Main Online Paper (Held On 09 April 2016)
A)
(A)-(iii), (B)-(i), (C)-(iv), (D)-(ii)
done
clear
B)
(A)-(ii), (B)-(i), (C)-(iv), (D)-(iii)
done
clear
C)
(A)-(iv), (B)-(i), (C)-(ii), (D)-(iii)
done
clear
D)
(A)-(ii), (B)-(iv), (C)-(i), (D)-(iii)
done
clear
View Answer play_arrow
-
question_answer6) The group of molecules having identical shape is :
JEE Main Online Paper (Held On 09 April 2016)
A)
\[PC{{l}_{5}},I{{F}_{5}},Xe{{O}_{2}}{{F}_{2}}\]
done
clear
B)
\[B{{F}_{3}},PC{{l}_{3}},Xe{{O}_{3}}\]
done
clear
C)
\[Cl{{F}_{3}},XeO{{F}_{2}},XeF_{3}^{+}\]
done
clear
D)
\[S{{F}_{4}},Xe{{F}_{4}},CC{{l}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer7) Which one of the following species is stable in aqueous solution?
JEE Main Online Paper (Held On 09 April 2016)
A)
\[MnO_{4}^{2-}\]
done
clear
B)
\[MnO_{4}^{3-}\]
done
clear
C)
\[C{{u}^{+}}\]
done
clear
D)
\[C{{r}^{2+}}\]
done
clear
View Answer play_arrow
-
question_answer8) For the reaction, \[A(g)+B(g)\to C(g)+D(g),\Delta {{H}^{0}}\]and\[\Delta {{S}^{0}}\] are, respectively,\[-29.8kJ\,mo{{l}^{-1}}\]and\[-0.100kJ\,{{K}^{-1}}mo{{l}^{-1}}\]at 298 K. The equilibrium constant for the reaction at 298 K is:
JEE Main Online Paper (Held On 09 April 2016)
A)
1
done
clear
B)
10
done
clear
C)
1.0 × 10.10
done
clear
D)
1.0 × 1010
done
clear
View Answer play_arrow
-
question_answer9) Assertion : Rayon is a semisynthetic polymer whose properties are better than natural cotton. Reason : Mechanical and aesthetic properties of cellulose can be improved by acetylation.
JEE Main Online Paper (Held On 09 April 2016)
A)
Both assertion and reason are correct, and the reason is the correct explanation for the assertion.
done
clear
B)
Both assertion and reason are incorrect.
done
clear
C)
Assertion is incorrect statement, but the reason is correct.
done
clear
D)
Both assertion and reason are correct, but the reason is not the correct explanation for the assertion.
done
clear
View Answer play_arrow
-
question_answer10) The hydrocarbon with seven carbon atoms containing a neopentyl and a vinyl group is :
JEE Main Online Paper (Held On 09 April 2016)
A)
4,4-dimethylpentene
done
clear
B)
2,2-dimethyl-4-pentene
done
clear
C)
Isopropyl-2-butene
done
clear
D)
2,2-dimethyl-3-pentene
done
clear
View Answer play_arrow
-
question_answer11) The gas evolved on heating \[C{{H}_{3}}MgBr\]in methanol is:
JEE Main Online Paper (Held On 09 April 2016)
A)
Propane
done
clear
B)
Ethane
done
clear
C)
HBr
done
clear
D)
Methane
done
clear
View Answer play_arrow
-
question_answer12) Identify the correct trend given below: (Atomic No.: Ti = 22, Cr = 24 and Mo = 42)
JEE Main Online Paper (Held On 09 April 2016)
A)
\[{{\Delta }_{o}}\]of\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}<{{[Mo{{({{H}_{2}}O)}_{6}}]}^{2+}}\]and\[{{\Delta }_{o}}\]of\[{{[Ti{{({{H}_{2}}O)}_{6}}]}^{3+}}>{{[Ti{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[{{\Delta }_{o}}\]of\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}>{{[Mo{{({{H}_{2}}O)}_{6}}]}^{2+}}\]and\[{{\Delta }_{o}}\]of\[{{[Ti{{({{H}_{2}}O)}_{6}}]}^{3+}}<{{[Ti{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
C)
\[{{\Delta }_{o}}\]of\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}>{{[Mo{{({{H}_{2}}O)}_{6}}]}^{2+}}\]and \[{{\Delta }_{o}}\]of\[{{[Ti{{({{H}_{2}}O)}_{6}}]}^{3+}}<{{[Ti{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
D)
\[{{\Delta }_{o}}\]of\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}<{{[Mo{{({{H}_{2}}O)}_{6}}]}^{2+}}\]and \[{{\Delta }_{o}}\]of\[{{[Ti{{({{H}_{2}}O)}_{6}}]}^{3+}}>{{[Ti{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
-
question_answer13) The most appropriate method of making egg-albumin sol is:
JEE Main Online Paper (Held On 09 April 2016)
A)
Keep the egg in boiling water for 10 minutes. After removing the shell, transfer the yellow part of the content to 100 mL of 5% w/V saline solution and homogenize with a mechanical shaker.
done
clear
B)
Break an egg carefully and transfer the transparent part of the content to 100 mL of 5% w/V saline solution and stir well.
done
clear
C)
Keep the egg in boiling water for 10 minutes. After removing the shell, transfer the white part of the content to 100 mL of 5% w/V saline solution and homogenize with a mechanical shaker.
done
clear
D)
Break an egg carefully and transfer only the yellow part of the content to 100 mL of 5% w/V saline solution and stir well.
done
clear
View Answer play_arrow
-
question_answer14) Which one of the following complexes will consume more equivalents of aqueous solution of \[Ag(N{{O}_{3}})\]?
JEE Main Online Paper (Held On 09 April 2016)
A)
\[N{{a}_{3}}[CrC{{l}_{6}}]\]
done
clear
B)
\[[Cr{{({{H}_{2}}O)}_{5}}Cl]C{{l}_{2}}\]
done
clear
C)
\[[Cr{{({{H}_{2}}O)}_{6}}]C{{l}_{3}}\]
done
clear
D)
\[[N{{a}_{2}}(CrC{{l}_{5}})({{H}_{2}}O)]\]
done
clear
View Answer play_arrow
-
question_answer15) At very high pressures, the compressibility factor of one mole of a gas is given by :
JEE Main Online Paper (Held On 09 April 2016)
A)
\[1+\frac{pb}{RT}\]
done
clear
B)
\[\frac{pb}{RT}\]
done
clear
C)
\[1-\frac{b}{(VRT)}\]
done
clear
D)
\[1-\frac{pb}{RT}\]
done
clear
View Answer play_arrow
-
question_answer16) A reaction at 1 bar is non-spontaneous at low temperature but becomes spontaneous at high temperature. Identify the correct statement about the reaction among the following:
JEE Main Online Paper (Held On 09 April 2016)
A)
Both\[\Delta H\] and \[\Delta S\] are positive.
done
clear
B)
\[\Delta H\]is negative while \[\Delta S\] is positive.
done
clear
C)
\[\Delta H\]is positive while\[\Delta S\]is negative.
done
clear
D)
Both \[\Delta S\]and \[\Delta S\] are negative.
done
clear
View Answer play_arrow
-
question_answer17) Which intermolecular force is most responsible in allowing xenon gas to liquefy?
JEE Main Online Paper (Held On 09 April 2016)
A)
Instantaneous dipole-induced dipole
done
clear
B)
Ionic
done
clear
C)
Ion-dipole
done
clear
D)
Dipole-dipole
done
clear
View Answer play_arrow
-
question_answer18) Identify the incorrect statement regarding heavy water:
JEE Main Online Paper (Held On 09 April 2016)
A)
It reacts with \[Ca{{C}_{2}}\]to produce \[{{C}_{2}}{{D}_{2}}\] and \[Ca{{(OD)}_{2}}.\]
done
clear
B)
It is used as a coolant in nuclear reactors.
done
clear
C)
It reacts with \[A{{l}_{4}}{{C}_{3}}\] to produce \[C{{D}_{4}}\] and \[Al{{(OD)}_{3}}.\]
done
clear
D)
It reacts with \[S{{O}_{3}}\]to form deuterated sulphuric acid \[({{D}_{2}}S{{O}_{4}}).\]
done
clear
View Answer play_arrow
-
question_answer19)
A particular adsorption process has the following characteristics:
|
(i) It arises due to vander Waals forces and
|
|
(ii) it is reversible. Identify the correct statement that describes the above adsorption process:
|
JEE Main Online Paper (Held On 09 April 2016)
A)
Enthalpy of adsorption is greater than \[100 \text{kJ}\,\text{mo}{{\text{l}}^{\text{-1}}}.\]
done
clear
B)
Adsorption is monolayer.
done
clear
C)
Adsorption increases with increase in temperature.
done
clear
D)
Energy of activation is low.
done
clear
View Answer play_arrow
-
question_answer20)
The plot shows the variation of .ln Kp versus temperature for the two reactions.
|
\[M(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}MO(s)\]and
|
|
\[C(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}CO(s)\]
|
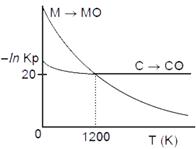 |
Identify the correct statement:
JEE Main Online Paper (Held On 09 April 2016)
A)
At T > 1200 K, carbon will reduce MO(s) to M(s).
done
clear
B)
At T < 1200 K, oxidation of carbon is unfavourable.
done
clear
C)
Oxidation of carbon is favourable at all temperatures.
done
clear
D)
At \[T<1200K,\] the reaction \[MO(s)+C(s)\to M(s)+CO(g)\]is spontaneous.
done
clear
View Answer play_arrow
-
question_answer21) BOD stands for:
JEE Main Online Paper (Held On 09 April 2016)
A)
Biochemical Oxygen Demand
done
clear
B)
Biochemical Oxidation Demand
done
clear
C)
Biological Oxygen Demand
done
clear
D)
Bacterial Oxidation Demand
done
clear
View Answer play_arrow
-
question_answer22) What will occur if a block of copper metal is dropped into a beaker containing a solution of\[1MZnS{{O}_{4}}?\]
JEE Main Online Paper (Held On 09 April 2016)
A)
The copper metal will dissolve and zinc metal will be deposited.
done
clear
B)
The copper metal will dissolve with evolution of oxygen gas.
done
clear
C)
The copper metal will dissolve with evolution of hydrogen gas.
done
clear
D)
No reaction will occur.
done
clear
View Answer play_arrow
-
question_answer23) The test to distinguish primary, secondary and tertiary amine is:
JEE Main Online Paper (Held On 09 April 2016)
A)
Mustard oil test
done
clear
B)
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}Cl\]
done
clear
C)
Sandmeyer's reaction
done
clear
D)
Carbylamine reaction
done
clear
View Answer play_arrow
-
question_answer24) The total number of orbitals associated with the principal quantum number 5 is:
JEE Main Online Paper (Held On 09 April 2016)
A)
5
done
clear
B)
20
done
clear
C)
25
done
clear
D)
10
done
clear
View Answer play_arrow
-
question_answer25) The correct order of the solubility of alkaline-earth metal sulphates in water is:
JEE Main Online Paper (Held On 09 April 2016)
A)
Mg < Sr < Ca < Ba
done
clear
B)
Mg > Ca> Sr > Ba
done
clear
C)
Mg > Sr > Ca > Ba
done
clear
D)
Mg < Ca < Sr < Ba
done
clear
View Answer play_arrow
-
question_answer26) An organic compound contains C, H and S. The minimum molecular weight of the compound containing 8% sulphur is: (atomic weight of S = 32 amu)
JEE Main Online Paper (Held On 09 April 2016)
A)
300 g \[\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
B)
400 g \[\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
C)
200 g \[\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
D)
600 g \[\text{mo}{{\text{l}}^{\text{-1}}}\]
done
clear
View Answer play_arrow
-
question_answer27) Bouveault-Blanc reduction reaction involves:
JEE Main Online Paper (Held On 09 April 2016)
A)
Reduction of an anhydride with \[LiAl{{H}_{4}}.\]
done
clear
B)
Reduction of an ester with \[Na/{{C}_{2}}{{H}_{5}}OH.\]
done
clear
C)
Reduction of a carbonyl compound with Na/Hg and HCl.
done
clear
D)
Reduction of an acyl halide with H2/Pd.
done
clear
View Answer play_arrow
-
question_answer28)
Consider the following sequence for aspartic acid: 
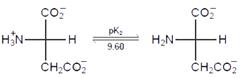 The pl (isoelectric point) of aspartic acid is:
JEE Main Online Paper (Held On 09 April 2016)
The pl (isoelectric point) of aspartic acid is:
JEE Main Online Paper (Held On 09 April 2016)
A)
5.74
done
clear
B)
3.65
done
clear
C)
2.77
done
clear
D)
1.88
done
clear
View Answer play_arrow
-
question_answer29) The amount of arsenic pentasulphide that can be obtained when 35.5 g arsenic acid is treated with excess \[{{H}_{2}}S\]in the presence of conc. HCl (assuming 100% conversion)
JEE Main Online Paper (Held On 09 April 2016)
A)
0.25 mol
done
clear
B)
0.125 mol
done
clear
C)
0.333 mol
done
clear
D)
0.50 mol
done
clear
View Answer play_arrow
-
question_answer30) The solubility of \[{{N}_{2}}\]in water at 300 K and 500 torr partial pressure is 0.01 g \[{{\text{L}}^{\text{-1}}}\]. The solubility (in g \[{{\text{L}}^{\text{-1}}}\]) at 750 torr partial pressure is :
JEE Main Online Paper (Held On 09 April 2016)
A)
0.02
done
clear
B)
0.015
done
clear
C)
0.0075
done
clear
D)
0.005
done
clear
View Answer play_arrow


 The pl (isoelectric point) of aspartic acid is:
JEE Main Online Paper (Held On 09 April 2016)
The pl (isoelectric point) of aspartic acid is:
JEE Main Online Paper (Held On 09 April 2016)
