A) \[IC{{l}_{5}}\]is trigonal bipyramidal and\[ICl_{4}^{-}\]is tetrahedral.
B) \[IC{{l}_{5}}\]is square pyramidal and\[ICl_{4}^{-}\]is tetrahedral.
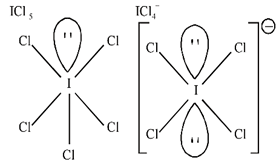
C) \[ICl_{5}^{{}}\]is square pyramidal and \[ICl_{4}^{-}\]is square planar.
D) Both are isostructural.
Correct Answer: C
Solution :
| Chemical species | Hybridization | Shape |
| \[ICl_{5}^{{}}\] | \[s{{p}^{3}}{{d}^{2}}\] | Square pyramidal |
| \[ICl_{4}^{-}\] | \[s{{p}^{3}}{{d}^{2}}\] | Square planar |
You need to login to perform this action.
You will be redirected in
3 sec
