A)
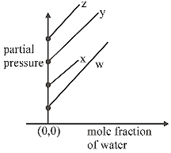
B)
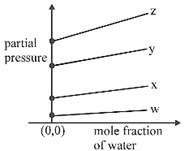
C)
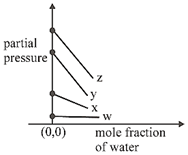
D)
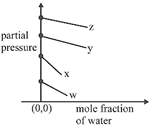
Correct Answer: C
Solution :
\[p={{k}_{H}}\times \left( \frac{{{n}_{gas}}}{{{n}_{{{H}_{2}}O}}+{{n}_{gas}}} \right)\] \[={{k}_{H}}\left( 1-\frac{{{n}_{{{H}_{2}}O}}}{{{n}_{{{H}_{2}}O}}+{{n}_{gas}}} \right)\] \[\Rightarrow \]\[p={{k}_{H}}-{{k}_{H}}\times {{\chi }_{{{H}_{2}}O}}\] \[p=(-{{k}_{H}})\times {{\chi }_{{{H}_{2}}O}}+{{k}_{H}}\]You need to login to perform this action.
You will be redirected in
3 sec
