A) \[Asp<Gly<Lys<Arg\]
B) \[Arg<Lys<Gly<Asp\]
C) \[Gly<Asp<Arg<Lys\]
D) \[Asp<Gly<Arg<Lys\]
Correct Answer: A
Solution :
Acidic strength \[\propto \,\,\frac{1}{pKa}\,\,\propto \,\,EWG\] \[pKa\,\,=\,\,-\,\log \,\,Ka\]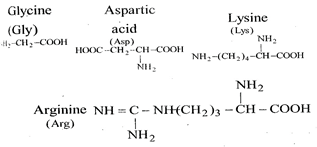 Acidic strength in solution \[\Rightarrow \text{ }ASP\text{ }>\text{ }Gly\text{ }>Lys\text{ }>\text{ }Arg\] pKa order should be \[\Rightarrow \text{ }Asp\text{ }<\text{ }Gly\text{ }<\text{ }Lys\text{ }<Arg\] [Though data suggest this is order of Isoelectric pH value]
Acidic strength in solution \[\Rightarrow \text{ }ASP\text{ }>\text{ }Gly\text{ }>Lys\text{ }>\text{ }Arg\] pKa order should be \[\Rightarrow \text{ }Asp\text{ }<\text{ }Gly\text{ }<\text{ }Lys\text{ }<Arg\] [Though data suggest this is order of Isoelectric pH value]
You need to login to perform this action.
You will be redirected in
3 sec
