A) \[\sigma {{*}^{2}}{{P}_{z}}\]
B) \[{{\pi }^{2}}{{P}_{y}}\]
C) \[\pi *\,{{\,}^{2}}{{P}_{x}}\]
D) \[\pi {{\,}^{2}}{{P}_{x}}\]
Correct Answer: C
Solution :
A complex having strong field ligand has tendency to absorb light of highest energy. Among the three complexes. \[{{\left[ Co{{(N{{H}_{3}})}_{6}} \right]}^{+3}}\]will absorb radiation of highest energy and least wavelength. \[{{\left[ Co{{(N{{H}_{3}})}_{5}}{{H}_{2}}O \right]}^{+3}}\]has field weaker than the above compound and therefore absorb radiation of lesser energy and more wavelength. \[{{\left[ CoCl{{(N{{H}_{3}})}_{5}} \right]}^{+2}}\]has the weakest field and therefore will absorb light of least energy and highest wavelength. Strength of ligand \[N{{H}_{3}}>{{H}_{2}}O>Cl.\] Molecular orbital diagram of \[{{O}_{2}}\]is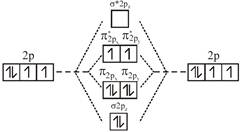
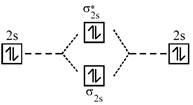
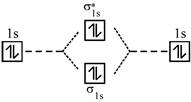 An incoming electron will go in \[\pi _{2{{p}_{x}}}^{*}\] orbital.
An incoming electron will go in \[\pi _{2{{p}_{x}}}^{*}\] orbital.
You need to login to perform this action.
You will be redirected in
3 sec
