A) Y unit
B) Y/R unit
C) YR unit
D) -Y unit.
Correct Answer: A
Solution :
According to Arrhenius equation, \[A{{e}^{-}}^{\frac{{{E}_{a}}}{RT}}\]or\[\ln \,k=\ln \,A-\frac{{{E}_{a}}}{RT}\] Comparing the above equation with straight line equation, \[y=mx+c,\]we get, slope \[(m)=-{{E}_{a}}\]ln k Intercept = ln A Thus, slope should be \[{{E}_{a}}=Y.\]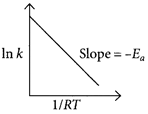
You need to login to perform this action.
You will be redirected in
3 sec
