 [JEE Main Online Paper Held On 12-Jan-2019 Evening]
[JEE Main Online Paper Held On 12-Jan-2019 Evening]
A) \[{{10}^{-4}}{{s}^{-1}}\]
B) \[4\times {{10}^{-4}}{{s}^{-1}}\]
C) \[{{10}^{-6}}{{s}^{-1}}\]
D) \[2\times {{10}^{-4}}{{s}^{-1}}\]
Correct Answer: A
Solution :
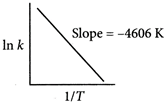
From Arrhenius equation, In\[k=\ln \,A-\frac{{{E}_{a}}}{RT}\] Slope \[=\frac{-{{E}_{a}}}{R}=-4606K\] or,\[\ln =\frac{{{k}_{2}}}{{{k}_{1}}}=\frac{{{E}_{a}}}{R}\left( \frac{{{T}_{2}}-{{T}_{1}}}{{{T}_{1}}{{T}_{2}}} \right)\] \[\ln =\frac{{{k}_{2}}}{{{10}^{-5}}}=4606\left( \frac{500-400}{500\times 400} \right)\] \[\ln \frac{{{k}_{2}}}{{{10}^{-5}}}=2.303;\frac{{{k}_{2}}}{{{10}^{-5}}}=anti\ln (2.303)\] \[{{k}_{2}}=1\times {{10}^{-4}}{{s}^{-1}}\]You need to login to perform this action.
You will be redirected in
3 sec
