A) \[B{{F}_{3}}>I_{3}^{-}>P{{F}_{3}}>N{{H}_{3}}\]
B) \[I_{3}^{-}>N{{H}_{3}}>P{{F}_{3}}>B{{F}_{3}}\]
C) \[B{{F}_{3}}>N{{H}_{3}}>P{{F}_{3}}>I_{3}^{-}\]
D) \[I_{3}^{-}>B{{F}_{3}}>N{{H}_{3}}>P{{F}_{3}}\]
Correct Answer: D
Solution :
\[lp-lp>{{l}_{p}}-bp>bp-bp\] Due to \[Dp-lp\] repulsion in \[P{{F}_{3}},F-P-F\] cmgle will be less than that of \[B{{F}_{3}}\], \[\therefore B{{F}_{3}}<P{{F}_{3}}\] \[B{{F}_{3}}<N{{H}_{3}}\], as the geometry of \[B{{F}_{3}}\] is triagonal planar while that \[N{{H}_{3}}\] is tetrahedral As the Fluorine atom pulls the lone pair of electron on P atom, \[lp-bp\] repulsion is more pre dominant. Thus \[N{{H}_{3}}>P{{F}_{3}}\] thus,\[B{{F}_{3}}>N{{H}_{3}}>P{{F}_{3}}\] \[I_{3}^{-}\]ion linear. This bond angle is \[180{}^\circ \]. \[\therefore \] Is \[I_{3}^{-}>B{{F}_{3}}>N{{H}_{3}}>P{{F}_{3}}\]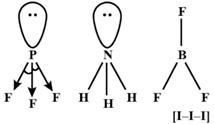
You need to login to perform this action.
You will be redirected in
3 sec
