A) \[\text{(i)-2, (ii)-2}\]
B) \[(i)->2,\,\,(ii)->2\]
C) \[(i)->2,\,\,(ii)-<2\]
D) \[(i)-<2,\,\,(ii)<2\]
Correct Answer: A
Solution :
As in the resonance structure of hydrogen azide, it can be seen than number of bond is \[\le 2\] for bond \[(i)\] Hence its bond order will be\[<2\] Whereas for bond\[(ii)\], number of bond\[\ge 2\] Thus its bond order will be \[>2\].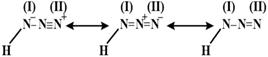
You need to login to perform this action.
You will be redirected in
3 sec
