A) \[5,3,0\]
B) \[5,2,0\]
C) \[4,2,2\]
D) \[4,4,0\]
Correct Answer: A
Solution :
In \[Xe{{O}_{3}}{{F}_{2}}\], the number of bond pair(s), \[\pi -\]bond(s) and lone pair(s) on Xe atom are \[5,3,0\]respectively. There are three \[Xe=O\] double bonds and two \[Xe-F\]single bonds. All the valence electrons of Xe are involved in bonding.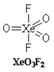
You need to login to perform this action.
You will be redirected in
3 sec
