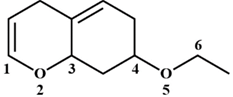
A) \[O2-C3\]
B) \[O5-C6\]
C) \[C4-O5\]
D) \[C1-O2\]
Correct Answer: B
Solution :
On the treatment of the given compound with a strong acid, the most susceptible site for bond cleavage is \[O5-C6\]. The lone pair of electrons on \[O2\] is involved in resonance with\[C=C\]. Hence, \[O2\] will not be protonated. The lone pair of electrons on \[O5\] is not involved in resonance with \[C=C\]. Hence, \[O5\]will be protonated. Chloride ion will then attack least substituted C atom \[(C6)\]You need to login to perform this action.
You will be redirected in
3 sec
