A) \[10.9g\]
B) \[109.0g\]
C) \[9.81g\]
D) 98.1 g
Correct Answer: C
Solution :
9.65 ampere current was passed for 1.0 hour (3600 seconds) Number of moles of electrons passed \[=\frac{IA\times t(s)}{96500}=\frac{96.5A\times 3600s}{96500}=0.36\]moles 4 moles of electrons will reduce 1 mole of nitrobenzene to p-aminophenol. 0.36 moles of electrons will reduce\[\frac{0.36}{4}=0.09\] moles of nitrobenzene to p-aminophenol. p-aminophenol molar mass 109.14 g/mol Mass of p-aminophenol obtained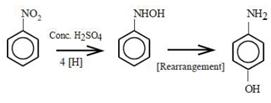
You need to login to perform this action.
You will be redirected in
3 sec
