A) 2, 4, 6 - Trinitrophenol
B) Benzoic acid
C) o-Nitrophenol
D) Benzene sulphonic acid
Correct Answer: B
Solution :
Due to intramolecular hydrogen bonding this will not be soluble in sodium bicarbonate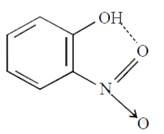
You need to login to perform this action.
You will be redirected in
3 sec
