A) 0.24
B) 0.15
C) 0.32
D) 0.08
Correct Answer: B
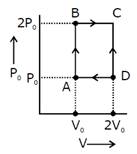
Solution :
\[w={{P}_{0}}{{V}_{0}}\] Heat given \[={{Q}_{AB}}={{Q}_{BC}}\] \[=n{{C}_{V}}d{{T}_{AB}}+m{{C}_{P}}d{{T}_{BC}}\] \[=\frac{3}{2}(nR{{T}_{B}}-nR{{T}_{A}})+\frac{5}{2}(nR{{T}_{C}}-nR{{T}_{B}})\] \[=\frac{3}{2}(2{{P}_{0}}{{V}_{0}}-{{P}_{0}}{{V}_{0}})+\frac{5}{2}(4{{P}_{0}}{{V}_{0}}-2{{P}_{0}}V)\] \[=\frac{13}{2}{{P}_{0}}{{V}_{0}}\] \[n=\frac{\text{w}}{\text{Qgiven}}=\frac{2}{13}=0.15\]You need to login to perform this action.
You will be redirected in
3 sec
