A) \[\left( \frac{e}{2m} \right)\frac{{{\operatorname{n}}^{2}}h}{2\pi }\]
B) \[\left( \frac{e}{m} \right)\frac{\operatorname{n}h}{2\pi }\]
C) \[\left( \frac{e}{2m} \right)\frac{\operatorname{n}h}{2\pi }\]
D) \[\left( \frac{e}{m} \right)\frac{{{\operatorname{n}}^{2}}h}{2\pi }\]
Correct Answer: C
Solution :
As, \[i=\frac{e}{T}\]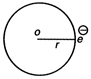 and magnetic moment \[M=iA\] \[(\because \,A=\pi {{r}^{2}})\] \[\therefore \] \[M=\frac{e}{T}\cdot \,\pi {{r}^{2}}\] ?(i) Now, \[T=\frac{2\pi r}{v}\] It becomes, \[M=\frac{\frac{e}{2\pi r}\cdot \,\pi {{r}^{2}}}{v}=\frac{evr}{2}\] ?(ii) Also, \[mvr=\frac{nh}{2\pi }\] \[vr=\frac{nh}{2\pi m}\] Putting this value in Eq. (ii), we get \[M=\frac{e\cdot nh}{2.2\pi m}=\left( \frac{e}{2m} \right)\frac{nh}{2\pi }\]
and magnetic moment \[M=iA\] \[(\because \,A=\pi {{r}^{2}})\] \[\therefore \] \[M=\frac{e}{T}\cdot \,\pi {{r}^{2}}\] ?(i) Now, \[T=\frac{2\pi r}{v}\] It becomes, \[M=\frac{\frac{e}{2\pi r}\cdot \,\pi {{r}^{2}}}{v}=\frac{evr}{2}\] ?(ii) Also, \[mvr=\frac{nh}{2\pi }\] \[vr=\frac{nh}{2\pi m}\] Putting this value in Eq. (ii), we get \[M=\frac{e\cdot nh}{2.2\pi m}=\left( \frac{e}{2m} \right)\frac{nh}{2\pi }\]
You need to login to perform this action.
You will be redirected in
3 sec
