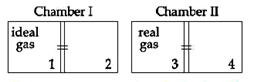
 There are two identical chambers, completely thermally insulated from surroundings. Both chambers have a partition wall dividing the chambers in two compartments. Compartment 1 is filled with an ideal gas and compartment 3 id filled with a real gas. Compartments 2 and 4 are vacuum. A small hole (orifice) is made in the partition walls and the gases are allowed to expand in vacuum. Statement-1: No change in the temperature of the gas takes place when ideal as expands in vacuum. However, the temperature of real gas goes down (cooling) when it expands it expands in vacuum. Statement-2: The internal energy of an ideal gas is only kinetic. The internal energy of a real gas is kinetic as well as potential.
JEE Main Online Paper (Held On 09 April 2013)
There are two identical chambers, completely thermally insulated from surroundings. Both chambers have a partition wall dividing the chambers in two compartments. Compartment 1 is filled with an ideal gas and compartment 3 id filled with a real gas. Compartments 2 and 4 are vacuum. A small hole (orifice) is made in the partition walls and the gases are allowed to expand in vacuum. Statement-1: No change in the temperature of the gas takes place when ideal as expands in vacuum. However, the temperature of real gas goes down (cooling) when it expands it expands in vacuum. Statement-2: The internal energy of an ideal gas is only kinetic. The internal energy of a real gas is kinetic as well as potential.
JEE Main Online Paper (Held On 09 April 2013)
A) Statement 1 is false and Statement 2is true
B) Statement 1 and Statement 2 bath are true. Statement 2 is the correct explanation of Statement 1.
C) Statement 1 is true and Statement 2 if false.
D) Statement 1 and Statement 2 both are true. Statement 2 is not the correct explanation of Statement 1.
Correct Answer: C
Solution :
Intermolecular distance in ideal gases is assume to be large as compared to real one. Hence, the internal energy of an ideal gas is PE and a real gas is only kinetic as well as potential.You need to login to perform this action.
You will be redirected in
3 sec
