A) \[\operatorname{X}e{{\operatorname{OF}}_{2}}\]
B) \[\operatorname{X}e{{\operatorname{O}}_{3}}{{F}_{2}}\]
C) \[\operatorname{F}\operatorname{X}e\operatorname{O}{{\operatorname{SO}}_{2}}\operatorname{F}\]
D) \[{{\left[ \operatorname{X}\operatorname{e}{{\operatorname{F}}_{8}} \right]}^{2-}}\]
Correct Answer: B
Solution :
Trigonal bipyramidal geometry is shown by \[Xe{{O}_{3}}{{F}_{2}}\]. Hybridization on Xe atom, \[H=\frac{1}{2}(V+M-C+A)=\frac{1}{2}(8+2-0+0)\] \[=\frac{10}{2}=5=s{{p}^{3}}d\] Trigonal bipyramidal geometry.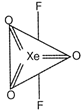 Structure of \[Xe{{O}_{3}}{{F}_{2}}\] molecule
Structure of \[Xe{{O}_{3}}{{F}_{2}}\] molecule
You need to login to perform this action.
You will be redirected in
3 sec
