A) \[{{p}^{{}^{3}/{}_{2}}}\]
B) \[{{p}^{3}}\]
C) \[{{p}^{{}^{2}/{}_{3}}}\]
D) \[{{p}^{2}}\]
Correct Answer: C
Solution :
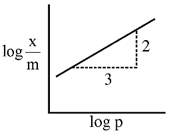
\[\frac{x}{m}=K.{{p}^{1/n}}\] \[\therefore \]\[\log \frac{x}{m}=\log K+\frac{1}{n}.\operatorname{logP}\] slope \[=\frac{1}{n}=\frac{2}{3}\] \[\therefore \]\[\frac{x}{m}=K.{{p}^{2/3}}\] Correct option : (c)You need to login to perform this action.
You will be redirected in
3 sec
