 [JEE Main 9-4-2019 Afternoon]
[JEE Main 9-4-2019 Afternoon]
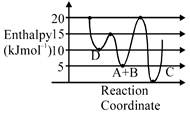
A) C is the thermodynamically stable product.
B) Formation of A and B from C has highest enthalpy of activation.
C) D is kinetically stable product.
D) Activation enthalpy to form C is \[5kJ\,mo{{l}^{-1}}\]less than that to form D.
Correct Answer: D
Solution :
\[A+B\to C+D\]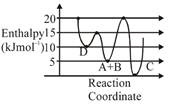 Activation enthalpy for \[C=205=15kJ/mol\] Activation enthalpy for \[D=155=10kJ/mol\]
Activation enthalpy for \[C=205=15kJ/mol\] Activation enthalpy for \[D=155=10kJ/mol\]
You need to login to perform this action.
You will be redirected in
3 sec
