| (Given : \[h=6.63\times {{10}^{34\text{ }}}Js;c=3\times {{10}^{8}}m{{s}^{1}}\]) |
A) 9.4 nm
B) 12.3 nm
C) 10.8 nm
D) 11.4 nm
Correct Answer: C
Solution :
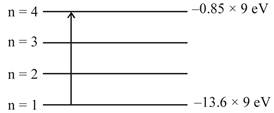 \[\Delta E=\frac{hc}{\lambda }\] \[13.6\times 9-0.85\times 9=\frac{hc}{\lambda }\] \[\lambda =\frac{hc}{9\times (13.6-0.85)eV}\] \[=\frac{1240eV.nm}{9\times 12.75eV}\] \[\lambda =10.8nm\]
\[\Delta E=\frac{hc}{\lambda }\] \[13.6\times 9-0.85\times 9=\frac{hc}{\lambda }\] \[\lambda =\frac{hc}{9\times (13.6-0.85)eV}\] \[=\frac{1240eV.nm}{9\times 12.75eV}\] \[\lambda =10.8nm\]
You need to login to perform this action.
You will be redirected in
3 sec
