[A]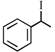 |
[B]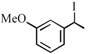 |
[C]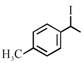 |
[D]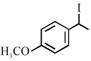 |
A) \[(A)<(B)<(C)<(D)\]
B) \[(B)<(A)<(D)<(C)\]
C) \[(B)<(A)<(C)<(D)\]
D) \[(A)<(B)<(D)<(C)\]
Correct Answer: C
Solution :
Rate of \[{{S}_{N'}}\]is directly proposional to stability of first formed carbocation so answer is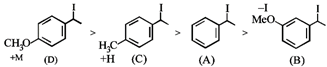
You need to login to perform this action.
You will be redirected in
3 sec
