A) \[1.68\times {{10}^{6}}J\]
B) \[1.71\times {{10}^{7}}J\]
C) \[1.51\times {{10}^{5}}J\]
D) 1\[1.67\times {{10}^{5}}J\]
Correct Answer: D
Solution :
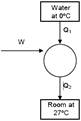 heat required to freeze 5 kg water \[=5\times 336\times {{10}^{3}}\] \[=168\times {{10}^{3}}\]Joule \[\Rightarrow {{Q}_{1}}=1680\,\,KJ\] for carnot's cycle \[\frac{{{Q}_{2}}}{{{Q}_{1}}}=\frac{{{T}_{2}}}{{{T}_{1}}}\] \[\frac{{{Q}_{2}}}{1680}=\frac{300}{273}\] \[{{Q}_{2}}=1680\times \frac{300}{273}KJ\] \[W-{{Q}_{2}}-Q{{ & }_{1}}\] \[=1680\left( \frac{300}{273}-1 \right)\] \[=\frac{1680\times 27}{273}\times {{10}^{3}}J\] \[=166.15\times {{10}^{3}}J\] \[=166.15\times {{10}^{5}}KJ\]
heat required to freeze 5 kg water \[=5\times 336\times {{10}^{3}}\] \[=168\times {{10}^{3}}\]Joule \[\Rightarrow {{Q}_{1}}=1680\,\,KJ\] for carnot's cycle \[\frac{{{Q}_{2}}}{{{Q}_{1}}}=\frac{{{T}_{2}}}{{{T}_{1}}}\] \[\frac{{{Q}_{2}}}{1680}=\frac{300}{273}\] \[{{Q}_{2}}=1680\times \frac{300}{273}KJ\] \[W-{{Q}_{2}}-Q{{ & }_{1}}\] \[=1680\left( \frac{300}{273}-1 \right)\] \[=\frac{1680\times 27}{273}\times {{10}^{3}}J\] \[=166.15\times {{10}^{3}}J\] \[=166.15\times {{10}^{5}}KJ\]
You need to login to perform this action.
You will be redirected in
3 sec
