(I) 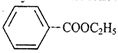 |
| (II) |
| (III) |
| (IV) |
A) \[III>II>IV>I\]
B) \[IV>II>III>I\]
C) \[III>II>I>IV\]
D) \[II>III>I>IV\]
Correct Answer: C
Solution :
Rate of alkaline Hydrolysis of ester occur through nucleophillic substitution by addition elimination in which attack of nucleophile is RDS Rate of SNAE \[\propto \] the charge on Alyl carbon so rate of alkaline Hcldrolysis is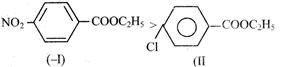
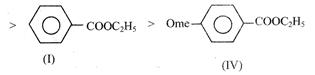
You need to login to perform this action.
You will be redirected in
3 sec
