A) 1
B) 2
C) 3
D) 4
Correct Answer: A
Solution :
Total electrons \[=2\times 7-1=13\] \[\therefore \]Number of electrons in \[{{\sigma }_{2p}}=1\]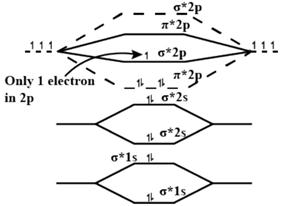
You need to login to perform this action.
You will be redirected in
3 sec
