A)
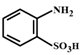
B)
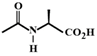
C)
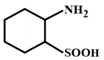
D)
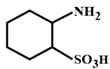
Correct Answer: B
Solution :
The compound in option (B) will not exist in zwitter ionic form at \[\text{pH=7}\] as N is in the form of amide group and not in the form of amine. Hence, at \[\text{pH=7}\], amide N will not be protonated.You need to login to perform this action.
You will be redirected in
3 sec
