A) \[B{{(C{{H}_{3}})}_{3}}\]
B) \[NaH\]
C) \[N{{F}_{3}}\]
D) \[P{{H}_{3}}\]
Correct Answer: A
Solution :
(ref. image) is a Lewis acid. It has empty orbital to accept electron pair.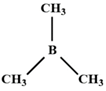
You need to login to perform this action.
You will be redirected in
3 sec
