| (A) |
| (B) |
| (C) |
(D) 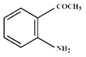 |
A) (D)<(C)<(B)<(A)
B) (A)<(D)<(B)<(C)
C) (A)<(B)<(C)<(D)
D) (A)<(D)<(C)<(B)
Correct Answer: B
Solution :
Aromatic diazonium salts are more stable than aliphatic diazonium salts. The stability of aryl diazonium salts is due to resonance. Electron donating substituents increase electron density on benzene ring. They increase the stability of diazonium salts. Electron withdrawing substituents decrease electron density on benzene ring. They decrease the stability of diazonium salts. \[-COC{{H}_{3}}\] group is electron withdrawing and hence, (d) is less stable than (b). Although \[-O-COC{{H}_{3}}\]is electron donating substituent, but it is present in meta position. Hence, it will not have significant effect on stability. The increasing order of diazotisation is (a)<(d)<(b)<(c).You need to login to perform this action.
You will be redirected in
3 sec
