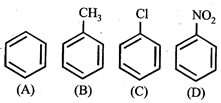
 In the above compounds correct order of reactivity in electrophilic substitution reactions will be:
JEE Main Online Paper ( Held On 25 April 2013 )
In the above compounds correct order of reactivity in electrophilic substitution reactions will be:
JEE Main Online Paper ( Held On 25 April 2013 )
A) \[b>a>c>d\]
B) \[d>c>b>a\]
C) \[a>b>c>d\]
D) \[b>c>a>d\]
Correct Answer: A
Solution :
- CF and\[-C{{H}_{3}}\] groups are o and p directing. They are electron releasing due to + E and + M effects. Further since' such groups increase electron density in the nucleus, they facilitate further electrophi-lic substitution and hence known as activating group. The activating effect of these groups is in order of\[-C{{H}_{3}}>-X\] but chlorine exceptionally deactive the ring due -to strong -1 effect. Hence, it is difficult to carry out substitution in chlorobenzene than in benzene. Further \[-N{{O}_{2}}\] is a deactivating group hence deactivates the benzene nucleus, i.e. hinder the further substitution. Thus nitrobenzene undergo electrophilic substitution with a great difficulty hence the correct order will be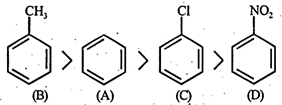
You need to login to perform this action.
You will be redirected in
3 sec
