 JEE Main Online Paper (Held On 26-May-2012)
JEE Main Online Paper (Held On 26-May-2012)
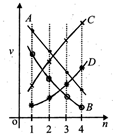
A) B
B) D
C) C
D) A
Correct Answer: A
Solution :
Velocity of electron in \[{{n}^{th}}\] orbit of hydrogen atom is given by :\[{{V}_{n}}=\frac{2\pi KZ{{e}^{2}}}{nh}\]Substituting the values we get,\[{{V}_{n}}=\frac{2.2\times {{10}^{6}}}{n}m/s\]or\[{{V}_{n}}\propto \frac{1}{n}\] As principal quantum number increases, velocity decreases.You need to login to perform this action.
You will be redirected in
3 sec
