A) \[O_{2}^{-}\] or \[O_{2}^{+}\]
B) \[{{O}_{2}},O_{2}^{-}\] or \[O_{2}^{+}\]
C) \[{{O}_{2}}\] or \[O_{2}^{+}\]
D) \[{{O}_{2}}\] or \[O_{2}^{-}\]
Correct Answer: A
Solution :
[a] In case of \[{{O}_{2}}\]: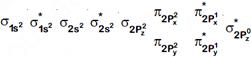
| Species | No. of unpaired e- |
| \[{{O}_{2}}\] | 2 |
| \[O_{2}^{-}\] | 1 |
| \[O_{2}^{+}\] | 1 |
| \[O_{2}^{2-}\] | 0 |
You need to login to perform this action.
You will be redirected in
3 sec
