A) \[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]
B) \[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
C) \[cis[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
D) trans\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
Correct Answer: C
Solution :
Complex \[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]have two G.I. which are optically inactive due to presence of plane of symmetry. Complex \[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]also have two optically inactive geometrical isomers due to presence of plane of symmetry. Complex cis \[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\] is optically active due to formation of non-super imposable mirror image.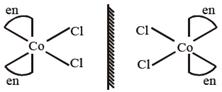 trans\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]Complex trans\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\] is optically inactive. (en = ethylenediamine)
trans\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]Complex trans\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\] is optically inactive. (en = ethylenediamine)
You need to login to perform this action.
You will be redirected in
3 sec
