 JEE Main Solved Paper-2017
JEE Main Solved Paper-2017
A) \[r=\frac{3}{4}\]
B) \[r=\frac{1}{3}\]
C) \[r=\frac{4}{3}\]
D) \[r=\frac{2}{3}\]
Correct Answer: B
Solution :
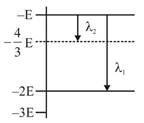
\[\Delta E=\frac{hC}{\lambda }\] For \[{{\lambda }_{1}}\]\[-E-(-2E)=\frac{hC}{{{\lambda }_{1}}}\] \[{{\lambda }_{1}}=\frac{hC}{E}\] For\[{{\lambda }_{2}}\] \[-E-\left( -\frac{4E}{3} \right)=\frac{hC}{{{\lambda }_{2}}}\] \[{{\lambda }_{2}}=\frac{3hC}{E}\] \[\frac{{{\lambda }_{1}}}{{{\lambda }_{2}}}=r=\frac{1}{3}\]You need to login to perform this action.
You will be redirected in
3 sec
