| A system goes from A to B via two processes and II as shown in figure. If |
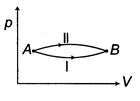 |
A) \[\Delta {{U}_{1}}=\Delta {{U}_{2}}\]
B) relation between\[\Delta {{U}_{1}}\]and\[\Delta {{U}_{2}}\]cannot be determined
C) \[\Delta {{U}_{2}}>\Delta {{U}_{1}}\]
D) \[\Delta {{U}_{2}}<\Delta {{U}_{1}}\]
Correct Answer: A
Solution :
| [a] The change in internal energy does not depend upon path followed by the process. It only depends on initial and final states. |
| Hence, |
You need to login to perform this action.
You will be redirected in
3 sec
