A) \[\frac{n_{1}^{2}T_{1}^{2}+n_{2}^{2}T_{2}^{2}+n_{3}^{2}T_{3}^{2}}{{{n}_{1}}{{T}_{1}}+{{n}_{2}}{{T}_{2}}+{{n}_{3}}{{T}_{3}}}\]
B) \[\frac{{{T}_{1}}+{{T}_{2}}+{{T}_{3}}}{3}\]
C) \[\frac{{{n}_{1}}{{T}_{1}}+{{n}_{2}}{{T}_{2}}+{{n}_{3}}{{T}_{3}}}{{{n}_{1}}+{{n}_{2}}+{{n}_{3}}}\]
D) \[\frac{{{n}_{1}}T_{1}^{2}+{{n}_{2}}T_{2}^{2}+{{n}_{3}}T_{3}^{2}}{{{n}_{1}}{{T}_{1}}+{{n}_{2}}{{T}_{2}}+{{n}_{3}}{{T}_{3}}}\]
Correct Answer: C
Solution :
[c] 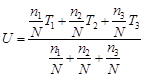 |
| |
You need to login to perform this action.
You will be redirected in
3 sec
