A) \[{{P}_{1}}<{{P}_{2}}\]
B) \[{{P}_{1}}>{{P}_{2}}\]
C) \[{{P}_{1}}={{P}_{2}}\]
D) all of the above are correct
Correct Answer: A
Solution :
From Boyles law \[P\propto \frac{1}{V}\] (at constant T)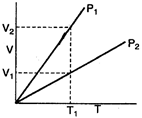 at a constant temperature \[{{T}_{1}}\], we can see, that Volume corresponding pressure \[{{P}_{1}}\] > volume corresponding to \[{{P}_{2}}\] \[\therefore \] \[{{P}_{1}}<{{P}_{2}}\]
at a constant temperature \[{{T}_{1}}\], we can see, that Volume corresponding pressure \[{{P}_{1}}\] > volume corresponding to \[{{P}_{2}}\] \[\therefore \] \[{{P}_{1}}<{{P}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
