A) trigonal bipyramidal
B) tetrahedral
C) pentagonal bipyramidal
D) square planar
Correct Answer: A
Solution :
\[{}_{15}P=1{{s}^{2}},\,2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{3}}3{{d}^{0}}\] P in excited state =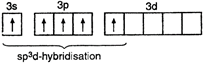 \[P{{F}_{5}}=\begin{matrix} \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, & \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, & \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, & \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, & \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, \\ \end{matrix}\]
\[P{{F}_{5}}=\begin{matrix} \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, & \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, & \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, & \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, & \underset{{{F}_{{{(2px)}^{1}}}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{3}}d)}^{1}}}}\,}}\, \\ \end{matrix}\] 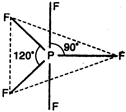 Thus, due to \[s{{p}^{3}}d\text{-}\]hybridization, \[P{{F}_{5}}\]molecule has trigonal bipyramidal geometry.
Thus, due to \[s{{p}^{3}}d\text{-}\]hybridization, \[P{{F}_{5}}\]molecule has trigonal bipyramidal geometry.
You need to login to perform this action.
You will be redirected in
3 sec
